Relationship Between the Thickness of the Coracoid Process and Latarjet Graft Positioning—An Anatomical Study on 70 Embalmed Scapulae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bone Limb Upper
Shoulder Pectoral girdle (shoulder girdle) Scapula Acromioclavicular joint proximal end of Humerus Clavicle Sternoclavicular joint Bone: Upper limb - 1 Scapula Coracoid proc. 3 angles Superior Inferior Lateral 3 borders Lateral angle Medial Lateral Superior 2 surfaces 3 processes Posterior view: Acromion Right Scapula Spine Coracoid Bone: Upper limb - 2 Scapula 2 surfaces: Costal (Anterior), Posterior Posterior view: Costal (Anterior) view: Right Scapula Right Scapula Bone: Upper limb - 3 Scapula Glenoid cavity: Glenohumeral joint Lateral view: Infraglenoid tubercle Right Scapula Supraglenoid tubercle posterior anterior Bone: Upper limb - 4 Scapula Supraglenoid tubercle: long head of biceps Anterior view: brachii Right Scapula Bone: Upper limb - 5 Scapula Infraglenoid tubercle: long head of triceps brachii Anterior view: Right Scapula (with biceps brachii removed) Bone: Upper limb - 6 Posterior surface of Scapula, Right Acromion; Spine; Spinoglenoid notch Suprspinatous fossa, Infraspinatous fossa Bone: Upper limb - 7 Costal (Anterior) surface of Scapula, Right Subscapular fossa: Shallow concave surface for subscapularis Bone: Upper limb - 8 Superior border Coracoid process Suprascapular notch Suprascapular nerve Posterior view: Right Scapula Bone: Upper limb - 9 Acromial Clavicle end Sternal end S-shaped Acromial end: smaller, oval facet Sternal end: larger,quadrangular facet, with manubrium, 1st rib Conoid tubercle Trapezoid line Right Clavicle Bone: Upper limb - 10 Clavicle Conoid tubercle: inferior -
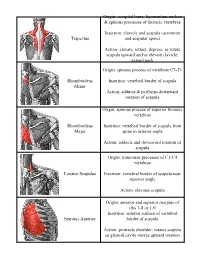
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Shoulder Shoulder
SHOULDER SHOULDER ⦿ Connects arm to thorax ⦿ 3 joints ◼ Glenohumeral joint ◼ Acromioclavicular joint ◼ Sternoclavicular joint ⦿ https://www.youtube.com/watch?v=rRIz6oO A0Vs ⦿ Functional Areas ◼ scapulothoracic ◼ scapulohumeral SHOULDER MOVEMENTS ⦿ Global Shoulder ⦿ Arm (Shoulder Movement Joint) ◼ Elevation ◼ Flexion ◼ Depression ◼ Extension ◼ Abduction ◼ Abduction ◼ Adduction ◼ Adduction ◼ Medial Rotation ◼ Medial Rotation ◼ Lateral Rotation ◼ Lateral Rotation SHOULDER MOVEMENTS ⦿ Movement of shoulder can affect spine and rib cage ◼ Flexion of arm Extension of spine ◼ Extension of arm Flexion of spine ◼ Adduction of arm Ipsilateral sidebending of spine ◼ Abduction of arm Contralateral sidebending of spine ◼ Medial rotation of arm Rotation of spine ◼ Lateral rotation of arm Rotation of spine SHOULDER GIRDLE ⦿ Scapulae ⦿ Clavicles ⦿ Sternum ⦿ Provides mobile base for movement of arms CLAVICLE ⦿ Collarbone ⦿ Elongated S shaped bone ⦿ Articulates with Sternum through Manubrium ⦿ Articulates with Scapula through Acromion STERNOCLAVICULAR JOINT STERNOCLAVICULAR JOINT ⦿ Saddle Joint ◼ Between Manubrium and Clavicle ⦿ Movement ◼ Flexion - move forward ◼ Extension - move backward ◼ Elevation - move upward ◼ Depression - move downward ◼ Rotation ⦿ Usually movement happens with scapula Scapula Scapula ● Flat triangular bone ● 3 borders ○ Superior, Medial, Lateral ● 3 angles ○ Superior, Inferior, Lateral ● Processes and Spine ○ Acromion Process, Coracoid Process, Spine of Scapula ● Fossa ○ Supraspinous, Infraspinous, Subscapularis, Glenoid SCAPULA -

Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K
Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K. Eichinger, MD Shoulder instability may be caused from congenital deformity, recurrent overuse activity, and/or traumatic dislocation. Surgical stabilization of the glenohumeral joint is indicated after conservative treatment fails and recurrent instability/subluxation continues. A number of different surgical procedures may be indicated in this situation, often divided into soft tissue or bony procedures. Shoulder Instability – Soft Tissue: Surgical reconstruction targeting the glenohumeral joint’s soft tissues for shoulder instability, typically involves labral repairs, the most common being the Bankart repair. A Bankart lesion typically occurs from an anterior-inferior dislocation of the humerus, tearing the labrum from it’s attachment to the glenoid, thereby detaching the inferior gleno-humeral ligament (IGHL). Surgical management of this revolves around labral repair to reattach the IGHL under appropriate tension. This may be accomplished either arthroscopically or through an open approach.1 Most traumatic glenohumeral dislocations may not only cause a Bankart lesion, but may create impression fractures in the postero-superior humeral head termed Hill-Sachs lesions.2 An adverse effect from this procedure includes suturing the capsule too tightly, causing a shortening of the capsule, and thus decreasing the external rotation allowed at the glenohumeral joint. Other complications are extremely rare, but may include axillary nerve damage, subscapularis rupture (seen only in open repairs performed with subscapularis detachment and repair), and recurrent instability. If there is bony deficiency in the glenoid, which represents 20% or more of the antero-inferior glenoid, it is biomechanically impossible to restore the same stability and is therefore more prone to recurrent instability and failure. -
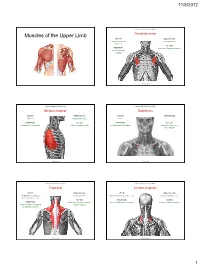
Muscles of the Upper Limb.Pdf
11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles of the Upper Limb Pectoralis minor ORIGIN: INNERVATION: anterior surface of pectoral nerves ribs 3 – 5 ACTION: INSERTION: protracts / depresses scapula coracoid process (scapula) (Anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Serratus anterior Subclavius ORIGIN: INNERVATION: ORIGIN: INNERVATION: ribs 1 - 8 long thoracic nerve rib 1 ---------------- INSERTION: ACTION: INSERTION: ACTION: medial border of scapula rotates scapula laterally inferior surface of scapula stabilizes / depresses pectoral girdle (Lateral view) (anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Trapezius Levator scapulae ORIGIN: INNERVATION: ORIGIN: INNERVATION: occipital bone / spinous accessory nerve transverse processes of C1 – C4 dorsal scapular nerve processes of C7 – T12 ACTION: INSERTION: ACTION: INSERTION: stabilizes / elevates / retracts / upper medial border of scapula elevates / adducts scapula acromion / spine of scapula; rotates scapula lateral third of clavicle (Posterior view) (Posterior view) 1 11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles Moving Arm Rhomboids Pectoralis major (major / minor) ORIGIN: INNERVATION: ORIGIN: INNERVATION: spinous processes of C7 – T5 dorsal scapular nerve sternum / clavicle / ribs 1 – 6 dorsal scapular nerve INSERTION: ACTION: INSERTION: ACTION: medial border of scapula adducts / rotates scapula intertubucular sulcus / greater tubercle flexes / medially rotates / (humerus) adducts -

Applied Anatomy of the Shoulder Girdle
Applied anatomy of the shoulder girdle CHAPTER CONTENTS intra-articular disc, which is sometimes incomplete (menis- Osteoligamentous structures . e66 coid) and is subject to early degeneration. The joint line runs obliquely, from craniolateral to caudomedial (Fig. 2). Acromioclavicular joint . e66 Extra-articular ligaments are important for the stability of Sternoclavicular joint . e66 the joint and to keep the movements of the lateral end of the Scapulothoracic gliding mechanism . e67 clavicle within a certain range. Together they form the roof of Costovertebral joints . e68 the shoulder joint (see Standring, Fig. 46.14). They are the coracoacromial ligament – between the lateral border of the Muscles and their innervation . e69 coracoid process and the acromion – and the coracoclavicular Anterior aspect of the shoulder girdle . e69 ligament. The latter consists of: Posterior aspect of the shoulder girdle . e69 • The trapezoid ligament which runs from the medial Mobility of the shoulder girdle . e70 border of the coracoid process to the trapezoid line at the inferior part of the lateral end of the clavicle. The shoulder girdle forms the connection between the spine, • The conoid ligament which is spanned between the base the thorax and the upper limb. It contains three primary artic- of the coracoid process and the conoid tubercle just ulations, all directly related to the scapula: the acromioclavicu- medial to the trapezoid line. lar joint, the sternoclavicular joint and the scapulothoracic Movements in the acromioclavicular joint are directly related gliding surface (see Putz, Fig. 289). The shoulder girdle acts as to those in the sternoclavicular joint and those of the scapula. a unit: it cannot be functionally separated from the secondary This joint is also discussed inthe online chapter Applied articulations, i.e. -

Pectoral Girdle Anatomy & Physiology Fundamentals > Skeletal > Skeletal
Pectoral Girdle Anatomy & Physiology Fundamentals > Skeletal > Skeletal Pectoral girdle • Connects the upper limb to the axial skeleton and provides muscle attachment sites for both the arm and back • Comprises the clavicle (collarbone) and the scapula (shoulder blade) Clavicle Anatomy: • Sternal end - flat medial end, articulates with manubrium of sternum • Conoid tubercle - small bony projection where the conoid ligament attaches • Acromion end – rounded lateral end, articulates with acromion of scapula • Costal tuberosity (aka impression) - provides an attachment site for the costoclavicular ligament • Superior surface - smooth • Inferior surface - rough due to muscular attachments Key Points: • Cannot withstand heavy forces; fractures are common and can cause damage to the underlying brachial plexus and associated vessels. • The clavicle is one of the first bones to begin ossification during fetal development, but is the last to complete ossification, during the mid-twenties. • It is the only long bone that ossifies intramembraneously. Scapula Anatomy: • Supraspinous fossa - superior to the spine • Infraspinous fossa - inferior to the spine • Body – triangular surface of scapula • Superior angle – projects superomedially • Inferior angle - projects inferiorly • Lateral angle – projects laterally; includes the glenoid cavity 1 / 2 • Superior border - extends from the glenoid cavity to the superior angle • Lateral (aka axillary) border - extends from the inferior angle to the lateral angle • Medial (aka vertebral) border - extends from -

Coracoid Process Fracture with Acromioclavicular Joint
(aspects of sports medicine) Coracoid Process Fracture With Acromioclavicular Joint Separation in an American Football Player: A Case Report and Literature Review Matthew DiPaola, MD, and Paul Marchetto, MD ABSTRACT 336 collegiate American football to palpation over the AC joint and Coracoid process fractures are rare players had a history of shoulder coracoid process. In addition, he had and few cases have been report- injury, 41% of which were acromio- pain with cross-body adduction of the ed in the orthopedic literature. In clavicular (AC) joint injuries. None symptomatic arm. Anteroposterior this article, we report the case of of these football players had a his- (AP), lateral, and axillary radiographs an American football player with tory of coracoid fracture. of both shoulders were obtained. The a coracoid process fracture in the setting of acromioclavicular sep- Coracoid fractures and ipsilateral AP radiograph showed a nondis- aration and describe incidence, shoulder injuries often occur concur- placed fracture of the coracoid and mechanism of injury, and treatment. rently. Ogawa and colleagues1 found a grade II AC separation (Figures 1, Although rare, coracoid process that 37 of 67 coracoid fractures were 2). Radiographic examination of the fracture should be considered in the associated with ipsilateral AC dis- asymptomatic shoulder confirmed differential diagnosis for shoulder locations. With the incidence of AC that the growth plates in this region pain. Treatment varies according to fracture type. Based on our lit- erature review, we recommend that clinicians initially treat nondisplaced “There is evidence that, of the athletes coracoid fractures nonoperatively. who sustain this injury, football players oracoid process fractures perhaps are at highest risk.” are relatively rare and have been estimated to account for 3% to 13% injury as high as it is in the football were closed. -
Lab Manual Appendicular Skele
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the appendicular skeleton, which is formed from the pectoral and pelvic girdles and the upper and lower limbs. View Module 7.2 Axial and Appendicular Skeleton to highlight the bones of the appendicular skeleton and compare them to those of the axial skeleton. Examine Module 11.1 Appendicular Skeleton to view only the bones of the appendicular skeleton. In addition to learning about all the bones of the appendicular skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Proximal: Trochanter: Tubercle: Tuberosity: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the appendicular skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. -

Chapter 4 the Shoulder Girdle
Bones • Key bony landmarks – Manubrium – Clavicle Chapter 4 – Coracoid process The Shoulder Girdle – Acromion process – Glenoid fossa – Lateral border – Inferior angle – Medial border © McGraw-Hill Higher Education. All rights reserved. 4-1 © McGraw-Hill Higher Education. All rights reserved. 4-2 Bones Joints • Key bony landmarks • Shoulder girdle (scapulothoracic) – Acromion process – scapula moves on the rib cage – Glenoid fossa – joint motion occurs at sternoclavicular joint – Lateral border & to a lesser amount at the – Inferior angle acromioclavicular joint – Medial border – Superior angle – Spine of the scapula From Seeley RR, Stephens TD, Tate P; anatomy and physiology , ed 7, New York, 2006, McGraw-Hill © McGraw-Hill Higher Education. All rights reserved. 4-3 © McGraw-Hill Higher Education. All rights reserved. 4-4 Joints Joints • Sternoclavicular (SC) • Sternoclavicular (SC) – (multiaxial) arthrodial classification – Ligamentous support – Movements • anteriorly by the anterior SC ligament • anteriorly 15 degrees with protraction • posteriorly by the posterior SC ligament • posteriorly 15 degrees with retraction • costoclavicular & interclavicular • superiorly 45 degrees with elevation ligaments provide stability against • inferiorly 5 degrees with depression superior displacement © McGraw-Hill Higher Education. All rights reserved. 4-5 © McGraw-Hill Higher Education. All rights reserved. 4-6 1 Joints Joints • Acromioclavicular (AC) • Scapulothoracic – arthrodial classification – not a true synovial joint – 20- to 30-degree total -

Glenoid and Coracoid Dimensions and Their Implications in the Latarjet Operation: a Dry Bone Study
Research article East African Orthopaedic Journal GLENOID AND CORACOID DIMENSIONS AND THEIR IMPLICATIONS IN THE LATARJET OPERATION: A DRY BONE STUDY K.C. Lakati, FCS Orth (ECSA), Department of Human Anatomy, Egerton University, Kenya, B.M. Ndeleva MMed (Mak), FCS Orth (ECSA), Department of Surgery and Orthopaedics, Kenyatta University, Nairobi, Kenya, R.S. Kenani, A. Kiprotich and M. Injete , Egerton University, Njoro, Kenya Corresponding author: Dr. K. C. Lakati, P.O. Box 15711-20100, Nakuru, Kenya. Email: [email protected] ABSTRACT Background: The coracoid process is widely used as a graft in patients with recurrent anterior shoulder instability with significant glenoid bone defects. However, no local studies have determined the coracoid dimensions and correlated them to the glenoid dimensions. Objective: To measure the widest anteroposterior (AP) diameter of the glenoid cavity, the length, width and thickness of the coracoid process, compare these with other populations, determine the amount of coverage the thickness and width of the coracoid process can afford in case of bony glenoid deficiency and the adequacy of the coracoid process to safely accommodate fixation screws used in the Latarjet and congruent-arc Latarjet procedures. Methods: The dimensions were measured using digital vernier callipers on dried scapulae that were not deformed. The ratio between coracoid thickness and width to the glenoid AP diameter was determined as the percentage cover that particular dimension can provide to the deficient glenoid. Results: A total of 26 scapulae were obtained. Average AP diameter and height of the glenoid was 25.1mm and 36.2mm respectively. Average coracoid length, width and thickness was 22.3mm, 13.3mm and 7.7mm respectively. -

Upper Extremity Counterstrain Counterstrain Counterstrain
1 Upper Extremity Counterstrain Dan Williams, D.O. Board Certified Neuromusculoskeletal Medicine And Osteopathic Manipulation 2 Counterstrain • Osteopathic manipulation technique developed by Larry Jones, D.O. • Discovered by accident • Based upon finding tender points and then passive patient positioning to treat the tender point 3 Counterstrain Advantages • Easy to teach – Little need for biomechanics – Find tender point - Fold and hold • Easy to implement at the bedside • Great gateway technique for further study in osteopathic manipulation • Safe for most patients • Requires minimal patient cooperation 4 Counterstrain Advantages • Great for acute pain • Great for headaches • Great for sports injuries • Great for patients with soft tissue pain 5 Counterstrain Points • Have tissue texture change • Are tender to palpation • Frequently located in relationship to soft tissue structures • Can be used as a standalone technique • Frequently combined with other manipulative modalities 6 Principles of Counterstrain • Identify the tender point – May be based on pain pattern, regional scan, observation, etc. • Establish a pain scale (1-10) and stay on the tender point • Bring patient to text book treatment position • Patient is completely passive! 7 Principles of Counterstrain • Recheck tenderness of tender point – Want at least a 70% reduction • Continue to monitor the tender point • Hold with patient completely relaxed for 90 seconds 1 • Occasionally recheck that point is no longer tender 8 Principles of Counterstrain • While continuously monitoring