Polycyclic Organic Matter (POM)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactivity and Functionalization of Naphthalene and Anthracene Complexes of {Tpw(NO)(Pme3)}
Reactivity and Functionalization of Naphthalene and Anthracene Complexes of {TpW(NO)(PMe3)} Laura Jessica Strausberg Baltimore, Maryland B.A., Hollins University, 2008 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Chemistry University of Virginia July, 2013 ii Abstract Chapter 1 introduces the organic chemistry of aromatic hydrocarbons, with attention paid to regiochemical outcomes of organic reactions. The binding of naphthalene and anthracene to metal complexes is discussed, along with organic transformations they undergo as a result of their complexation. The previous work on osmium and rhenium complexes of naphthalene from the Harman group is explored. Finally, some spectroscopic techniques for exploring the chemistry of {TpW(NO)(PMe3)} complexes of naphthalene and anthracene are introduced. Chapter 2 discusses the highly distorted allyl complexes formed from {TpW(NO)(PMe3)} and the exploration of their origin. Attempts at stereoselectively deprotonating these cationic complexes is also discussed. 2 Chapter 3 describes our study of TpW(NO)(PMe3)(3,4-η -naphthalene)’s ability to undergo a Diels-Alder reaction with N-methylmaleimide. A solvent study suggested that this reaction proceeds by a concerted mechanism. To probe the mechanism further, we synthesized a series of methylated and methoxylated naphthalene complexes and measured their rates of reaction with N-methylmaleimide compared to the parent complex. We found that 1- substitution on the naphthalene increased the rate of cycloaddition, even if the substituent was in the unbound ring, while 2-substitution slowed the reaction rate when in the bound ring. This information is consistent with a concerted mechanism, as a 2-substituted product would be less able to isomerize to form the active isomer for the cycloaddition to occur. -

The Effects of Detritus Input on Soil Organic Matter Content and Carbon Dioxide Emission in a Central European Deciduous Forest
Acta Silv. Lign. Hung., Vol. 7 (2011) 87–96 The Effects of Detritus Input on Soil Organic Matter Content and Carbon Dioxide Emission in a Central European Deciduous Forest a* b c István FEKETE – Zsolt KOTROCZÓ – Csaba VARGA – b b Zsuzsa VERES – János Attila TÓTH a Institute of Environmental Science, College of Nyíregyháza, Nyíregyháza, Hungary b Department of Ecology, University of Debrecen, Debrecen, Hungary c Department of Land and Environmental Management, College of Nyíregyháza, Nyíregyháza, Hungary Abstract – A major objective of our research was to survey soil biological activity and organic matter content reduction in a Central European oak forest during treatments of various detritus inputs within the Síkf őkút DIRT ( Detritus Input and Removal Treatments ) Project. Beside the control, three detritus removal and two detritus duplication treatments were applied. Our examinations have proven that soil organic matter content declined relatively fast in detritus removal treatments. The reduction was especially remarkable in root detritus removal treatments, where – due to the lack of transpiration – soils were moister during the whole year than in the other treatments. The higher moisture content, despite of the reduction of detritus input, produced an intense soil respiration. This can be explained by the fact that decomposing organisms have increased the use of soil organic matter. Detritus input reduction had a significantly greater effect on soil respiration and organic matter content than detritus input duplication of the same extent. The latter did not cause any significant change compared to the control. litter manipulation / soil respiration / DIRT Project / humus content / climate change Kivonat – Az avarinput hatása a talaj szerves anyag tartalmára és szén-dioxid kibocsátására egy Közép-európai lombhullató erd őben. -

Crystal Structure of Methyl 10-(Pyridin-4-Yl)-Anthracene-9
Z. Kristallogr. NCS 2018; 233(3): 441–443 Xiang Huang and Da-Bin Shi* Crystal structure of methyl 10-(pyridin-4-yl)- anthracene-9-carboxylate, C21H15NO2 Table 1: Data collection and handling. Crystal: Block, colorless Size: 0.30 × 0.20 × 0.10 mm Wavelength: Mo Kα radiation (0.71073 Å) µ: 0.09 mm−1 Diffractometer, scan mode: Bruker SMART, φ and ω-scans θmax, completeness: 27.6°, >99% N(hkl)measured, N(hkl)unique, Rint: 9199, 3516, 0.027 Criterion for Iobs, N(hkl)gt: Iobs > 2 σ(Iobs), 2700 N(param)refined: 218 Programs: Bruker programs [1], SHELX [2, 3] before stirring for 2 h at 65 °C. Crude 10-bromo-anthracene- 9-carboxylic acid was precipitated by adding the acetic acid solution to 800 mL of ice/water slush, followed by suc- https://doi.org/10.1515/ncrs-2017-0334 tion filtration. The residue on the filter was dissolved in Received October 31, 2017; accepted February 20, 2018; available 500 mL of a 5% aquaeus solution of K2CO3 followed by online March 6, 2018 gravity filtration to remove undissolved side products such as 9,10-dibromoanthracene. The filtrate was acidified with Abstract concentrated HCl to precipitate crude 10-bromo-anthracene- Aba a = C21H15NO2, orthorhombic, 2 (no. 41), 22.149(2) Å, 9-carboxylic acid, which was recrystallized from 100 mL b = c = V = 3 Z = 13.2899(12) Å, 10.6679(10) Å, 3140.2(5) Å , 8, ethanol to yield 5.76 g of yellow needles. R F = wR F2 = T = gt( ) 0.0418, ref( ) 0.0953, 296(2) K. -

Fractionation and Characterization of Natural Organic Matter from Certain Rivers and Soils by Free-Flow Electrophoresis
Fractionation and Characterization of Natural Organic Matter from Certain Rivers and Soils by Free-Flow Electrophoresis GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1817-E Fractionation and Characterzation of Natural Organic Matter from Certain Rivers and Soils by Free-Flow Electrophoresis By J. A. LEENHEER and R. L. MALCOLM ORGANIC SUBSTANCES IN WATER GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1817-E UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1973 UNITED STATES DEPARTMENT OF THE INTERIOR ROGERS C. B. MORTON, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress catalog-card No. 73-600209 For sale by the Superintendent of Documents, U. S. Government Printing Office Washington, D. C. 20402 - Price 35 cents (paper cov«?r) Stock No. 2401-02400 CONTENTS Page Abstract ___________________________________________ El Introduction __________________________________ __ 1 Methods and materials __________________________________ 3 Sample preparation ______ _______ _ __ 3 Sample description _________________________________ 3 Separation procedure _______ ___________ __ 4 Assay of fractions ___________________________________ 4 Results and discussion _ _______________ 5 Conclusions ________________________________________ 12 References _____________________________________ __ 14 ILLUSTRATIONS Page FIGURES 1-3. Graphs showing: 1. Organic-carbon and optical-density electrophoretic fraction- ation curves for organic materials from soil and from river water ___________________________ E6 2. Organic-carbon and polysaccharide electrophoretic -

Minutes of the IUPAC Chemical Nomenclature and Structure Representation Division (VIII) Committee Meeting Boston, MA, USA, August 18, 2002
Minutes of the IUPAC Chemical Nomenclature and Structure Representation Division (VIII) Committee Meeting Boston, MA, USA, August 18, 2002 Members Present: Dr Stephen Heller, Prof Herbert Kaesz, Prof Dr Alexander Lawson, Prof G. Jeffrey Leigh, Dr Alan McNaught (President), Dr. Gerard Moss, Prof Bruce Novak, Dr Warren Powell (Secretary), Dr William Town, Dr Antony Williams Members Absent: Dr. Michael Dennis, Prof Michael Hess National representatives Present: Prof Roberto de Barros Faria (Brazil) The second meeting of the Division Committee of the IUPAC Division of Chemical Nomenclature and Structure Representation held in the Great Republic Room of the Westin Hotel in Boston, Massachusetts, USA was convened by President Alan McNaught at 9:00 a.m. on Sunday, August 18, 2002. 1.0 President McNaught welcomed the members to this meeting in Boston and offered a special welcome to the National Representative from Brazil, Prof Roberto de Barros Faria. He also noted that Dr Michael Dennis and Prof Michael Hess were unable to be with us. Each of the attendees introduced himself and provided a brief bit of background information. Housekeeping details regarding breaks and lunch were announced and an invitation to a reception from the U. S. National Committee for IUPAC on Tuesday, August 20 was noted. 2.0 The agenda as circulated was approved with the addition of a report from Dr Moss on the activity on his website. 3.0 The minutes of the Division Committee Meeting in Cambridge, UK, January 25, 2002 as posted on the Webboard (http://www.rsc.org/IUPAC8/attachments/MinutesDivCommJan2002.rtf and http://www.rsc.org/IUPAC8/attachments/MinutesDivCommJan2002.pdf) were approved with the following corrections: 3.1 The name Dr Gerard Moss should be added to the members present listing. -
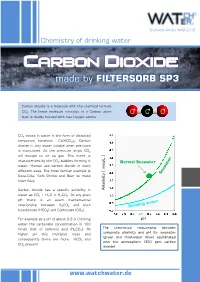
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Determining the Fraction of Organic Carbon RISC Nondefault Option
Indiana Department of Environmental Management Office of Land Quality 100 N. Senate Indianapolis, IN 46204-2251 GUIDANCE OLQ PH: (317) 232-8941 Indiana Department of Environmental Management Office of Land Quality Determining the Fraction of Organic Carbon RISC Nondefault Option Background Soil can be a complex mixture of mineral-derived compounds and organic matter. The ratios of each component can vary widely depending on the type of soil being investigated. Soil organic matter is a term used by agronomists for the total organic portion of the soil and is derived from decomposed plant matter, microorganisms, and animal residues. The decomposition process can create complex high molecular weight biopolymers (e.g., humic acid) as well as simpler organic compounds (decomposed lignin or cellulose). Only the simpler organic compounds contribute to the fraction of organic carbon. There is not a rigorous definition of the fraction of organic carbon (Foc). However, it can be thought of as the portion of the organic matter that is available to adsorb the organic contaminants of concern. The higher the organic carbon content, the more organic chemicals may be adsorbed to the soil and the less of those chemicals will be available to leach to the ground water. A nondefault option in the Risk Integrated System of Closure (RISC) is to use Foc in the Soil to Ground Water Partitioning Model to calculate a site specific migration to ground water closure level. In the Soil to Ground Water Partition Model, the coefficient, Kd, for organic compounds is the Foc multiplied by the chemical-specific soil organic carbon water partition coefficient, Koc. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Polycyclic Aromatic Hydrocarbon Structure Index
NIST Special Publication 922 Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899-0001 December 1997 revised August 2020 U.S. Department of Commerce William M. Daley, Secretary Technology Administration Gary R. Bachula, Acting Under Secretary for Technology National Institute of Standards and Technology Raymond G. Kammer, Director Polycyclic Aromatic Hydrocarbon Structure Index Lane C. Sander and Stephen A. Wise Chemical Science and Technology Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 This tabulation is presented as an aid in the identification of the chemical structures of polycyclic aromatic hydrocarbons (PAHs). The Structure Index consists of two parts: (1) a cross index of named PAHs listed in alphabetical order, and (2) chemical structures including ring numbering, name(s), Chemical Abstract Service (CAS) Registry numbers, chemical formulas, molecular weights, and length-to-breadth ratios (L/B) and shape descriptors of PAHs listed in order of increasing molecular weight. Where possible, synonyms (including those employing alternate and/or obsolete naming conventions) have been included. Synonyms used in the Structure Index were compiled from a variety of sources including “Polynuclear Aromatic Hydrocarbons Nomenclature Guide,” by Loening, et al. [1], “Analytical Chemistry of Polycyclic Aromatic Compounds,” by Lee et al. [2], “Calculated Molecular Properties of Polycyclic Aromatic Hydrocarbons,” by Hites and Simonsick [3], “Handbook of Polycyclic Hydrocarbons,” by J. R. Dias [4], “The Ring Index,” by Patterson and Capell [5], “CAS 12th Collective Index,” [6] and “Aldrich Structure Index” [7]. In this publication the IUPAC preferred name is shown in large or bold type. -

Determination of Petroleum Hydrocarbons in Sediments
UNITED NATIONS ENVIRONMENT PROGRAMME NOVEMBER 1992 Determination of petroleum hydrocarbons in sediments Reference Methods For Marine Pollution Studies No. 20 Prepared in co-operation with IOC IAEA UNEP 1992 ~ i - PREFACE The Regional Seas Programme was initiated by UNEP in 1974. Since then the Governing Conncil ofUNEP has repeatedly endorsed a regional approach to the control of marine pollution and the management of marine and coastal resources and has requested the development of regional action plans. The Regional Seas Progranune at present includes ten regions and has over 120 coastal States participating in it (1),(2). One of the basic components of the action plans sponsored by UNEP in the framework of the Regional Seas Programme is the assessment of the state of the marine em~ronment and of its resources, and of the sources and trends of the pollution, and the impact of pollution on human health, marine ecosystems and amenities. In order to assist those participating in this activity and to ensure that the data obtained through this assessment can be compared. on a world-wide basis and thns contribute to the Global Environment Monitoring System (GEMS) of UNEP, a set of Reference Methods and Guidelines for marine pollution studies is being developed as part of a programme of c9mprehensive technical support which includes the provision of expert advice, reference methods and materials, training and data quality assurance (3). The Methods are recommended to be adopted by Governments participating in tbe Regional Seas Programme. The methods and guidelines are prepared in co-operation with the relevant specialized bodies of the United Nations system as well as other organizations and are tested by a number of experts competent in the field relevant to the methods described. -

Construction of Novel Molecular Architectures from Anthracene Units and Acetylene Linkers*
Pure Appl. Chem., Vol. 84, No. 4, pp. 917–929, 2012. http://dx.doi.org/10.1351/PAC-CON-11-09-07 © 2012 IUPAC, Publication date (Web): 9 February 2012 Construction of novel molecular architectures from anthracene units and acetylene linkers* Shinji Toyota‡ Department of Chemistry, Faculty of Science, Okayama University of Science, 1-1 Ridaicho, Kita-ku, Okayama 700-0005, Japan Abstract: To create novel π-conjugated compounds, we constructed various molecular archi- tectures from anthracene units and acetylene linkers. Several cyclic oligomers ranging from dimers to dodecamers were synthesized by macrocyclization of acyclic precursors with metal-catalyzed coupling reactions. The structures, dynamic behavior, and spectroscopic fea- tures were greatly influenced by the number of anthracene units and the combination of building units and linkers. Optically active and circular dichroism (CD)-active enantiomers of some chiral cyclic oligomers were resolved by chiral high-performance liquid chromato - graphy (HPLC). Conformational analysis of hexamers and higher oligomers was performed with the aid of density functional theory (DFT) calculations. Acyclic oligomers underwent reversible folding–unfolding processes via photochemical and thermal reactions. These results suggest that transannular π–π interactions between anthracene units are important fac- tors in controlling the structural and spectroscopic properties and functions of π-conjugated compounds. The scope and perspectives of this molecular design are discussed on the basis of previous studies. Keywords: aromatic compounds; alkynes; π–π interactions; stereochemistry; structure. INTRODUCTION In the chemistry of aromatic compounds, oligomeric structures consisting of simple repeating units are fascinating motifs for the creation of new compounds. The merits of this molecular design are the acces- sibility to a large number of compounds from simple building units as well as the ease of tuning elec- tronic properties by structural modifications.