Mutation of Twins Encoding a Regulator of Protein Phosphatase 2A Leads to Pattern Duplication in Drosophila Imaginal Discs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix 1: Venus Missions
Appendix 1: Venus Missions Sputnik 7 (USSR) Launch 02/04/1961 First attempted Venus atmosphere craft; upper stage failed to leave Earth orbit Venera 1 (USSR) Launch 02/12/1961 First attempted flyby; contact lost en route Mariner 1 (US) Launch 07/22/1961 Attempted flyby; launch failure Sputnik 19 (USSR) Launch 08/25/1962 Attempted flyby, stranded in Earth orbit Mariner 2 (US) Launch 08/27/1962 First successful Venus flyby Sputnik 20 (USSR) Launch 09/01/1962 Attempted flyby, upper stage failure Sputnik 21 (USSR) Launch 09/12/1962 Attempted flyby, upper stage failure Cosmos 21 (USSR) Launch 11/11/1963 Possible Venera engineering test flight or attempted flyby Venera 1964A (USSR) Launch 02/19/1964 Attempted flyby, launch failure Venera 1964B (USSR) Launch 03/01/1964 Attempted flyby, launch failure Cosmos 27 (USSR) Launch 03/27/1964 Attempted flyby, upper stage failure Zond 1 (USSR) Launch 04/02/1964 Venus flyby, contact lost May 14; flyby July 14 Venera 2 (USSR) Launch 11/12/1965 Venus flyby, contact lost en route Venera 3 (USSR) Launch 11/16/1965 Venus lander, contact lost en route, first Venus impact March 1, 1966 Cosmos 96 (USSR) Launch 11/23/1965 Possible attempted landing, craft fragmented in Earth orbit Venera 1965A (USSR) Launch 11/23/1965 Flyby attempt (launch failure) Venera 4 (USSR) Launch 06/12/1967 Successful atmospheric probe, arrived at Venus 10/18/1967 Mariner 5 (US) Launch 06/14/1967 Successful flyby 10/19/1967 Cosmos 167 (USSR) Launch 06/17/1967 Attempted atmospheric probe, stranded in Earth orbit Venera 5 (USSR) Launch 01/05/1969 Returned atmospheric data for 53 min on 05/16/1969 M. -

The Van Allen Probes' Contribution to the Space Weather System
L. J. Zanetti et al. The Van Allen Probes’ Contribution to the Space Weather System Lawrence J. Zanetti, Ramona L. Kessel, Barry H. Mauk, Aleksandr Y. Ukhorskiy, Nicola J. Fox, Robin J. Barnes, Michele Weiss, Thomas S. Sotirelis, and NourEddine Raouafi ABSTRACT The Van Allen Probes mission, formerly the Radiation Belt Storm Probes mission, was renamed soon after launch to honor the late James Van Allen, who discovered Earth’s radiation belts at the beginning of the space age. While most of the science data are telemetered to the ground using a store-and-then-dump schedule, some of the space weather data are broadcast continu- ously when the Probes are not sending down the science data (approximately 90% of the time). This space weather data set is captured by contributed ground stations around the world (pres- ently Korea Astronomy and Space Science Institute and the Institute of Atmospheric Physics, Czech Republic), automatically sent to the ground facility at the Johns Hopkins University Applied Phys- ics Laboratory, converted to scientific units, and published online in the form of digital data and plots—all within less than 15 minutes from the time that the data are accumulated onboard the Probes. The real-time Van Allen Probes space weather information is publicly accessible via the Van Allen Probes Gateway web interface. INTRODUCTION The overarching goal of the study of space weather ing radiation, were the impetus for implementing a space is to understand and address the issues caused by solar weather broadcast capability on NASA’s Van Allen disturbances and the effects of those issues on humans Probes’ twin pair of satellites, which were launched in and technological systems. -

Storm Time Equatorial Magnetospheric Ion Temperature Derived from TWINS ENA Flux
Storm-time equatorial magnetospheric ion temperature derived from TWINS ENA flux R. M. Katus1,2,3, A. M. Keesee2, E. Scime2, M. W. and Liemohn3 1. Department of Mathematics, Eastern Michigan University, Ypsilanti, MI, USA 2. Department of Physics and Astronomy, West Virginia University, Morgantown, WVU, USA 3. Department of Climate and Space Sciences and Engineering, University of Michigan, Ann Arbor, MI, USA Submitted to: Journal of Geophysical Research Corresponding author email: [email protected] AGU index terms: 2788 Magnetic storms and substorms (4305, 7954) 4305 Space weather (2101, 2788, 7900) 4318 Statistical analysis (1984, 1986) 7954 Magnetic storms (2788) 2467 Plasma temperature and density Keywords: Magnetosphere, Geomagnetic Storms, Ion Temperature, Plasma Sheet, Space Weather Key points: • We derive and statistically examine storm-time equatorial magnetospheric ion temperatures from TWINS ENA flux This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/2016JA023824 This article is protected by copyright. All rights reserved. • The TWINS ion temperature data is validated using Geotail and LANL ion temperature data • For moderate to intense storms the widest (in MLT) peak in nightside ion temperature is found to exist near 12 RE This article is protected by copyright. All rights reserved. Abstract The plasma sheet plays an integral role in the transport of energy from the magnetotail to the ring current. We present a comprehensive study of the of equatorial magnetospheric ion temperatures derived from Two Wide-angle Imaging Neutral-atom Spectrometers (TWINS) Energetic Neutral Atom (ENA) measurements during moderate to intense (Dstpeak < -60 nT) storm times between 2009 and 2015. -

National Aeronautics and Space Administration
9 National Aeronautics and Space Administration Ross B. Garelick Bell American Institute of Aeronautics and Astronautics HIGHLIGHTS • The President’s Budget Request for NASA in FY 2014 is $17.7 billion, $1.3 billion above the estimated FY 2013 level and $84 million below the enacted FY 2012 level. • NASA’s FY 2013 appropriations suffered two across-the-board reductions, a 1.877% rescission in the FY 2013 Continuing Appropriations, P.L. 113-6, and the forced 5% sequester required by the Budget Control Act of 2011. The President’s Budget Request for FY 2014 does not account for the expected sequester required by the Budget Control Act of 2011 on FY 2014 appropriations. • The Orion Multi-Purpose Crew Vehicle and Space Launch System, scheduled for their first combined test launch in 2018, with a target crew launch in 2021, are slightly reduced in funding the President’s FY 2014 Budget Request. • As of May 25, 2012, with Space Exploration Technologies Corporation (SpaceX) having successfully completing its resupply mission to the ISS, commercial cargo has officially begun operations. SpaceX again resupplied the ISS on March 3, 3013. On April 22, 2013, Orbital Sciences Corporation successfully launched its Antares rocket from NASA’s Wallops Launch Facility on the coast of Virginia, another important milestone in creating a commercial market for cargo to the ISS. Orbital anticipates launching both the Antares rocket and the Cygnus capsule in the fall of 2013, and SpaceX, The Boeing Company, Sierra Nevada Corporation, and unfunded Blue Origin are working toward commercial crew missions to the ISS, with launches to begin in 2017. -

The Siamese Twins, the Bunker Family, and Nineteenth-Century U.S
American Family, Oriental Curiosity: The Siamese Twins, the Bunker Family, and Nineteenth-Century U.S. Society Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Joseph Andrew Orser Graduate Program in History The Ohio State University 2010 Dissertation Committee: Judy Tzu-Chun Wu, Adviser John Brooke Alan Gallay Copyright by Joseph Andrew Orser 2010 Abstract This dissertation examines the cultural and social spaces that conjoined brothers Chang and Eng Bunker occupied, interrogating the insights their lives offer into nineteenth-century ideas of race, class, gender, and respectability. Chang and Eng were conjoined twins of Chinese descent whose stage name, the Siamese Twins, derived from the country of their birth. The brothers toured the United States as “Oriental” curiosities from 1829 to 1839, and then settled in North Carolina as farmers, becoming slaveholders, marrying white sisters, and eventually fathering twenty-one children between them. In 1849, the twins returned to touring, this time taking two daughters along with them; until their deaths in 1874, Chang and Eng exhibited themselves and their offspring, touring as the Siamese Twins and Children. Through promotional literature, personal correspondence, visual images and newspaper reports, this work traces the evolution of public discourse about the twins and their families, contributing to other considerations of the twins and the course of American Orientalism. This dissertation goes further, however, by introducing early Asian Americans to considerations of the turbulent terrain of class and respectability in the 1830s and 1840s; the increasingly divisive debates over slavery, nativism, and sectionalism; and the tensions of national reunion in the years following the Civil War. -

Pace-Based Astromo 00000 O00000 O0 • 00000000 O00
Educational Product National Aeronautics Educators l Grades 5-8 EG-2001-01-122-HQ and Space Administration ! pace-Based Astromo 00000 O00000 O0 • 00000000 O00 ••• i ,iii- 0"• • • • • • • • • • Space-BasedAstronomy_An Educator Guide with • • Acth,ities for Science, Mathematics, and Technology • • Education is available in electronic format through • • NASA Spacelink--one of the Agency's electronic • • resources specifically developed for use by the educa- • • tional community. • • The system may be accessed at the following address: • • http://spacelink.nasa.gov • 00 • O O • O • • • • • • • • 00 • • • • • 00 • ASsPaCe- trono AN EDUCATOR GUIDE WITH ACTIVITIES ENCE MATHEMATICS AND LOGY EDU ',ATION NATIONALAERONAUTICSANDSPACEADMINISTRATIONI OFFICEOFHUMANRESOURCESANDEDUCATIONI EDUCATIONDIVISIONI OFFICEOFSPACESCIENCE Thispublicationis inthePublicDomainandis notprotectedbycopyright.Permissionis notrequiredforduplication. ",,,.._./ EG-2001-0!-122-HQ About the Cover Images 1. EIT304Aimage capturesa sweepingprominence--huge cloudsof relativelycooldenseplasma suspendedinthe Sun's hot, thin corona.At times, they can erupt,escapingthe Sun's atmosphere.Emissionin this spectral line shows the upper chro- mosphereat a temperatureof about 60,000 degreesK. SourceCredits:Solar & HeliosphericObservatory(SOHO).SOHOis a projectof internationalcooperationbetweenESAandNASA. 2. Thismosaicshowssome of the highest resolutionimages obtainedbythe Solid StateImaging(SSI)systemon NASA'sGalileo spacecraftduring its eleventhorbit around Jupiter.The sun illuminates the -

GRACE Mission Twins in Orbit Fly Linked by Microwaves Reveal Earth Mass in Motion — Ab Davis, GRACE Project Manager, JPL
GRACE Mission Twins in orbit fly linked by microwaves reveal Earth mass in motion — Ab Davis, GRACE Project Manager, JPL Goddard Space Flight Center Greenbelt, Maryland NP-2002-2-427-GSFC Acknowledgements Content Developed By: Alan Ward Designed By: Winnie Humberson Edited By: Margaret Baguio, Bill Bandeen, Charlotte Griner, Michael King, Margaret Srinivasan. Technical Input Provided By: Christoph Reigber, Rick Fitzgerald, Frank Flechtner, Bob Garnett, Marc Imhoff, Byron Tapley, Srinivas Bettadpur, John Ries, Don Chambers,Ab Davis, Mike Watkins. Front Cover Foreground: These images show three different views of the Earth’s geoid—a surface of equal gravitational potential that, over the ocean, closely follows the sea surface.The geoid was determined from data collected by previous satellite missions.Among these satellites is the Challenging Minisatellite Payload (CHAMP), the forerunner to GRACE, launched by the German Georesearch Center GeoForschungsZentrum Potsdam (GFZ). Similar products will be developed using data from GRACE with an expected improvement in accuracy of several orders of magnitude.These images were provided courtesy of GFZ. The illustration of the GRACE satellites was provided by Chris Meaney. Front Cover Background: This day/night image of the Earth was produced using a combination of data from the Moderate Resolution Imaging Spectrometer (MODIS) sensor aboard NASA’s Terra satellite (daytime portion), and the city lights image produced using Operational Line Scan (OLS) data from the Defense Meteorological Satellite.The daytime side of the Earth in this image was made using surface reflectance data from MODIS.The data was composited over a period of several months during the Northern Hemisphere of summer 2001. -
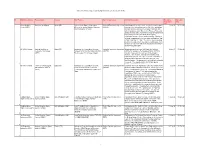
Active International Agreements by Signature Date (As of June 30, 2019)
Active International Agreements by Signature Date (as of June 30, 2019) No. NASA Installation Partner Name Country Title/Purpose Type of Agreement Activity Description Execution Expiration (Signature) Date Date Kennedy Space Government of Spain Spain (SP) Agreement on Space Cooperation Umbrella/Framework Agreement Authorization for, in case of an emergency, manned space 11-Jul-91 31-Dec-00 Center (KSC) Between the United States of America (UM/FW) vehicles of the United States to overfly, enter, and depart and the Kingdom of Spain Spanish air space and use the runways, taxiways, and other installations at the Moron de la Frontera, Rota, and Zaragoza bases; also, agreement to negotiate agreements in promising areas for joint efforts to strengthen cooperation in space science and technology. Dip notes entering the agreement into force were exchange on Sept 3, 1991, and May 12, 1994. The science and technology portion of this agreement was implemented by agreement SP0027 of 12/02/1991 with INTA and agreement SP0028 of 07/03/1992 with CDTI. 1 All NASA Centers National Institute for Spain (SP) Agreement on Cooperative Activities Umbrella/Framework Agreement Broad agreement between NASA and the National 2-Dec-91 31-Dec-00 Aerospace Technology Between NASA and the National Institute (UM/FW) Institute for Aerospace Technology of Spain (INTA) to (INTA) For Aerospace Technology of Spain consider cooperation in a variety of fields in Space Science, Earth Science, Aeronautics Research, and Exploration Systems. The agreement also establishes a group to discuss potential cooperative projects in the identified areas. The agreement is automatically extended each year. -

Atmospheric Science with Insight
Space Sci Rev (2018) 214:109 https://doi.org/10.1007/s11214-018-0543-0 Atmospheric Science with InSight Aymeric Spiga1,2 · Don Banfield3 · Nicholas A. Teanby4 · François Forget1 · Antoine Lucas5 · Balthasar Kenda5 · Jose Antonio Rodriguez Manfredi6 · Rudolf Widmer-Schnidrig7 · Naomi Murdoch8 · Mark T. Lemmon9 · Raphaël F. Garcia8 · Léo Martire8 · Özgür Karatekin10 · Sébastien Le Maistre10 · Bart Van Hove10 · Véronique Dehant10 · Philippe Lognonné5,2 · Nils Mueller11,12 · Ralph Lorenz13 · David Mimoun8 · Sébastien Rodriguez5,2 · Éric Beucler14 · Ingrid Daubar11 · Matthew P. Golombek11 · Tanguy Bertrand15 · Yasuhiro Nishikawa5 · Ehouarn Millour1 · Lucie Rolland16 · Quentin Brissaud17 · Taichi Kawamura 5 · Antoine Mocquet14 · Roland Martin18 · John Clinton19 · Éléonore Stutzmann5 · Tilman Spohn12 · Suzanne Smrekar11 · William B. Banerdt11 Received: 21 August 2018 / Accepted: 4 September 2018 © Springer Nature B.V. 2018 Abstract In November 2018, for the first time a dedicated geophysical station, the InSight lander, will be deployed on the surface of Mars. Along with the two main geophysical pack- The InSight Mission to Mars II Edited by William B. Banerdt and Christopher T. Russell B A. Spiga [email protected] 1 Laboratoire de Météorologie Dynamique (LMD/IPSL), Sorbonne Université, Centre National de la Recherche Scientifique, École Polytechnique, École Normale Supérieure, Paris, France 2 Institut Universitaire de France, Paris, France 3 Cornell Center for Astrophysics and Planetary Science, Cornell University, Ithaca, NY, -

Heliophysics at NASA and the Science of Space Weather
NASA Heliophysics Space Weather Science and Applications Program: Opportunity & Impact James Spann Space Weather Lead, Heliophysics Division 2019 Space Weather Workshop April 3, 2019 1 Opportunity & Impact • Sun-Earth-Solar System Coupled Science • Heliophysics System Observatory Evolution • Access to Space • Small Spacecraft • Launch Accessibility • National Space Weather Strategy and Action Plan • Space Weather Science and Applications (SWxSA) • Exploration Beyond Near-Earth Space 2 Opportunity & Impact 3 Opportunity & Impact 4 Overview • Space Weather Science and Applications Program - Research - Infrastructure - International and Interagency Partnerships • New Initiatives - Whole Helio Month campaigns - NASA Science Mission Directorate Rideshare policy - Heliophysics and the Lunar Gateway 5 NASA Heliophysics Research and Analysis* Supporting Research Applied Research Applied Living With a Star Space Weather Science and Applications Guest Investigator (Mission Data Driven) Fundamental Research Fundamental Grand Research Challenges – Science Centers + Theory Modelling * Not included are the investigations of the Heliophysics Technology Instrument Development for Science (HTIDeS) and the 18 operating missions 6 Space Weather Science and Applications (SWxSA) SWxSA Goals • Support investigations and facilitate related activities in concert with other Heliophysics Division programs, that significantly advance understanding and enable improved characterization and prediction of space weather • Transition tools, models, data, and knowledge -

From Cern to Aerospace
FROM CERN TO AEROSPACE Knowledge Transfer Accelerating Innovation FROM UNDERGROUND TO THE DEEP-SKY FROM CERN TO THE EXPLORATION OF THE UNIVERSE AND THE SEARCH FOR THE SECRETS OF MATTER FACE SIMILAR CHALLENGES LEADING TO COMMON SOLUTIONS. New Space revolution, clean aviation, multi-messenger astronomy… In recent times, the aeronautics and space sectors have been experiencing radical advancements which are of economic and scientific interest to CERN Member States. Thanks to its remarkable technologies, facilities and know-how, CERN plays a crucial role in aerospace. Aerospace and particle physics share many technical similarities in terms of operational environments. Both sectors need components capable of functioning in harsh radiation environments, extreme temperatures and high vacuum conditions. Moreover, they both need to be able to handle large amounts of data quickly and autonomously. Performed in orbit around the Earth to avoid atmospheric effects, fundamental physics missions in space have much in common with CERN’s underground experiments. Many synergies exist, in particular between particle and astroparticle physics - Radiation monitoring and dosimetry experiments, including but not limited to detection capabilities. - Rad-hard electronics and detectors - Low cost radiation tolerant systems design Maximising the positive impact of its research on society is - Superconducting and cryogenic systems integral to CERN’s mission. Aerospace applications carry strong - High performance thermal management opportunities in areas such as -

Evolution of Low‐Altitude and Ring Current ENA Emissions from a Moderate Magnetospheric Storm: Continuous and Simultaneous TWINS Observations P
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115, A11209, doi:10.1029/2010JA015429, 2010 Evolution of low‐altitude and ring current ENA emissions from a moderate magnetospheric storm: Continuous and simultaneous TWINS observations P. Valek,1,2 P. C. Brandt,3 N. Buzulukova,4 M.‐C. Fok,4 J. Goldstein,1,2 D. J. McComas,1,2 J. D. Perez,5 E. Roelof,3 and R. Skoug6 Received 5 March 2010; revised 30 June 2010; accepted 22 July 2010; published 13 November 2010. [1] The moderate storm of 22 July 2009 is the largest measured during the extended solar minimum between December 2006 and March 2010. We present observations of this storm made by the two wide‐angle imaging neutral‐atom spectrometers (TWINS) mission. The TWINS mission measures energetic neutral atoms (ENAs) using sensors mounted on two separate spacecrafts. Because the two spacecrafts’ orbital planes are significantly offset, the pair provides a nearly optimal combination of continuous magnetospheric observations from at least one of the TWINS platforms with several hours of simultaneous, dual‐platform viewing over each orbit. The ENA imaging study presented in this paper is the first reported magnetospheric storm for which both continuous coverage and stereoscopic imaging were available. Two populations of ENAs are observed during this storm. The first are emissions from the ring current and come from a parent population of trapped ions in the inner magnetosphere. The second, low‐altitude emissions (LAEs), are the result of precipitating ions which undergo multiple charge exchange and stripping collisions with the oxygen exosphere. The temporal evolution of this storm shows that the LAEs begin earlier and are the brightest emissions seen during the main phase, while later, during the recovery, the LAE is only as bright as the bulk ring current emissions.