Chapter 1: General Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upper Eocene) of the Sultanate of Oman
Pala¨ontol Z (2016) 90:63–99 DOI 10.1007/s12542-015-0277-1 RESEARCH PAPER Terrestrial and lacustrine gastropods from the Priabonian (upper Eocene) of the Sultanate of Oman 1 1 2 3 Mathias Harzhauser • Thomas A. Neubauer • Dietrich Kadolsky • Martin Pickford • Hartmut Nordsieck4 Received: 17 January 2015 / Accepted: 15 September 2015 / Published online: 29 October 2015 Ó The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Terrestrial and aquatic gastropods from the sparse non-marine fossil record of the Eocene in the Tethys upper Eocene (Priabonian) Zalumah Formation in the region. The occurrence of the genera Lanistes, Pila, and Salalah region of the Sultanate of Oman are described. The Gulella along with some pomatiids, probably related to assemblages reflect the composition of the continental extant genera, suggests that the modern African–Arabian mollusc fauna of the Palaeogene of Arabia, which, at that continental faunas can be partly traced back to Eocene time, formed parts of the southeastern Tethys coast. Sev- times and reflect very old autochthonous developments. In eral similarities with European faunas are observed at the contrast, the diverse Vidaliellidae went extinct, and the family level, but are rarer at the genus level. These simi- morphologically comparable Neogene Achatinidae may larities point to an Eocene (Priabonian) rather than to a have occupied the equivalent niches in extant environ- Rupelian age, although the latter correlation cannot be ments. Carnevalea Harzhauser and Neubauer nov. gen., entirely excluded. At the species level, the Omani assem- Arabiella Kadolsky, Harzhauser and Neubauer nov. gen., blages lack any relations to coeval faunas. -

The Significance of Shore Height in Intertidal Macrobenthic Seagrass
AQUATIC CONSERVATION: MARINE AND FRESHWATER ECOSYSTEMS Aquatic Conserv: Mar. Freshw. Ecosyst. 21: 614–624 (2011) Published online 20 October 2011 in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/aqc.1234 The significance of shore height in intertidal macrobenthic seagrass ecology and conservation R. S. K. BARNESa,b,* and M. D. F. ELLWOODb aKnysna Field Laboratory, Department of Zoology and Entomology, Rhodes University, P.O. Box 1186, Knysna, RSA bDepartment of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK ABSTRACT 1. Benthic faunal assemblages of an intertidal seagrass bed were sampled at three shore heights (LWN, MLW, LWS) at the mouth, mid-point and head of the Steenbok Channel in South Africa’s premier seagrass site, the warm-temperate Knysna estuarine bay, Garden Route National Park. 2. Faunal abundance, species richness, species diversity, and proportion of rare species were relatively uniform along the Channel, as were faunal abundance, species diversity and proportion of rare species down the shore. Overall species richness per station, however, was significantly lower at LWN than at either MLW or LWS, although the distribution of species richness over the shore did not depart from random at one of the three sites. Overall faunal abundance and those of individual component species were dispersed patchily through the bed. 3. The nature of the faunal assemblages present, however, varied significantly throughout the bed, both along the Channel at each shore-height horizon, and down the shore at each site. LWN assemblages formed a unit distinct from those at MLW and LWS. Overall, the shore-height axis accounted for 47% and the along-shore axis 28% of total assemblage variation. -
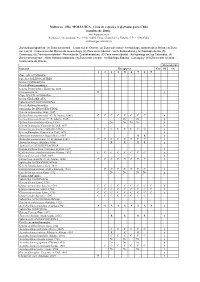
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

THREATENED and ENDANGERED SPECIES of NEW MEXICO 2008 Biennial Review and Recommendations
THREATENED AND ENDANGERED SPECIES OF NEW MEXICO 2008 BIENNIAL REVIEW DRAFT First Public Comment Period March 11, 2008 New Mexico Department of Game and Fish Conservation Services Division DRAFT 2008 Biennial Review of T & E Species of NM, 3/11/08 THREATENED AND ENDANGERED SPECIES OF NEW MEXICO 2008 Biennial Review and Recommendations Authority: Wildlife Conservation Act (17-2-37 through 17-2-46 NMSA 1978) EXECUTIVE SUMMARY: A total of 118 species and subspecies are on the 2008 list of threatened and endangered New Mexico wildlife. The list includes 2 crustaceans, 25 mollusks, 23 fishes, 6 amphibians, 15 reptiles, 32 birds and 15 mammals (Tables 1, 2). An additional 7 species of mammals has been listed as restricted to facilitate control of traffic in federally protected species. A species is endangered if it is in jeopardy of extinction or extirpation from the state; a species is threatened if it is likely to become endangered within the foreseeable future throughout all or a significant portion of its range in New Mexico. Species or subspecies of mammals, birds, reptiles, amphibians, fishes, mollusks, and crustaceans native to New Mexico may be listed as threatened or endangered under the Wildlife Conservation Act (WCA). During the Biennial Review, species may be upgraded from threatened to endangered, or downgraded from endangered to threatened, based upon data, views, and information regarding the biological and ecological status of the species. Investigations for new listings or removals from the list (delistings) can be undertaken at any time, but require additional procedures from those for the Biennial Review. The 2006 Biennial Review contained a recommendation for maintaining the status for 119 species and subspecies listed as threatened, endangered, or restricted under the WCA, and uplisting four species (Arizona grasshopper sparrow, Pecos bluntnose shiner, spikedace, and meadow jumping mouse ) from threatened to endangered and downlisting two species (shortneck snaggletooth and piping plover) from endangered to threatened. -

Liste De Référence Annotée Des Mollusques Continentaux De France Annotated Checklist of the Continental Molluscs from France
MalaCo Le journal de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Strasbourg / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIÉ / Montpellier / [email protected] Pour soumettre un article à MalaCo : 1ère étape – Le premier auteur veillera à ce que le manuscrit soit conforme aux recommandations aux auteurs (consultez le site www.journal-malaco.fr). Dans le cas contraire, la rédaction peut se réserver le droit de refuser l’article. 2ème étape – Joindre une lettre à l’éditeur, en document texte, en suivant le modèle suivant : "Veuillez trouvez en pièce jointe l’article rédigé par << mettre les noms et prénoms de tous les auteurs>> et intitulé : << mettre le titre en français et en anglais >> (avec X pages, X figures et X tableaux). Les auteurs cèdent au journal MalaCo (ISSN1778-3941) le droit de publication de ce manuscrit et ils garantissent que l’article est original, qu’il n’a pas été soumis pour publication à un autre journal, n’a pas été publié auparavant et que tous sont en accord avec le contenu." 3ème étape – Envoyez par voie électronique le manuscrit complet (texte et figures) en format .doc et la lettre à l’éditeur à : [email protected]. Pour les manuscrits volumineux (>5 Mo), envoyez un courriel à la même adresse pour élaborer une procédure FTP pour le dépôt du dossier final. -

Distribution and Habitats of the Alien Invader Freshwater Snail Physa Acuta in South Africa
Distribution and habitats of the alien invader freshwater snail Physa acuta in South Africa KN de Kock* and CT Wolmarans School of Environmental Sciences and Development, Zoology, Potchefstroom Campus of the North-West University, Private Bag X6001, Potchefstroom 2520, South Africa Abstract This article focuses on the geographical distribution and habitats of the invader freshwater snail species Physa acuta as reflected by samples taken from 758 collection sites on record in the database of the National Freshwater Snail Collection (NFSC) at the Potchefstroom Campus of the North-West University. This species is currently the second most widespread 1 alien invader freshwater snail species in South Africa. The 121 different loci ( /16- degree squares) from which the samples were collected, reflect a wide but discontinuous distribution mainly clustered around the major ports and urban centres of South Africa. Details of each habitat as described by collectors during surveys were statistically analysed, as well as altitude and mean annual air temperatures and rainfall for each locality. This species was reported from all types of water-bodies rep- resented in the database, but the largest number of samples was recovered from dams and rivers. Chi-square and effect size values were calculated and an integrated decision tree constructed from the data which indicated that temperature, altitude and types of water-bodies were the important factors that significantly influenced the distribution ofP. acuta in South Africa. Its slow progress in invading the relatively undisturbed water-bodies in the Kruger National Park as compared to the recently introduced invader freshwater snail species, Tarebia granifera, is briefly discussed. -

WCM 2001 Abstract Volume
Abstracts Council of UNITAS MALACOLOGICA 1998-2001 World Congress of President: Luitfried SALVINI-PLAWEN (Wien/Vienna, Austria) Malacology Secretary: Peter B. MORDAN (London, England, UK) Treasurer: Jackie VAN GOETHEM (Bruxelles/Brussels, Belgium) 2001 Members of Council: Takahiro ASAMI (Matsumoto, Japan) Klaus BANDEL (Hamburg, Germany) Yuri KANTOR (Moskwa/Moscow, Russia) Pablo Enrique PENCHASZADEH (Buenos Aires, Argentinia) John D. TAYLOR (London, England, UK) Vienna, Austria Retired President: Rüdiger BIELER (Chicago, USA) 19. – 25. August Edited by Luitfried Salvini-Plawen, Janice Voltzow, Helmut Sattmann and Gerhard Steiner Published by UNITAS MALACOLOGICA, Vienna 2001 I II Organisation of Congress Symposia held at the WCM 2001 Organisers-in-chief: Gerhard STEINER (Universität Wien) Ancient Lakes: Laboratories and Archives of Molluscan Evolution Luitfried SALVINI-PLAWEN (Universität Wien) Organised by Frank WESSELINGH (Leiden, The Netherlands) and Christiane TODT (Universität Wien) Ellinor MICHEL (Amsterdam, The Netherlands) (sponsored by UM). Helmut SATTMANN (Naturhistorisches Museum Wien) Molluscan Chemosymbiosis Organised by Penelope BARNES (Balboa, Panama), Carole HICKMAN Organising Committee (Berkeley, USA) and Martin ZUSCHIN (Wien/Vienna, Austria) Lisa ANGER Anita MORTH (sponsored by UM). Claudia BAUER Rainer MÜLLAN Mathias BRUCKNER Alice OTT Thomas BÜCHINGER Andreas PILAT Hermann DREYER Barbara PIRINGER Evo-Devo in Mollusca Karl EDLINGER (NHM Wien) Heidemarie POLLAK Organised by Gerhard HASZPRUNAR (München/Munich, Germany) Pia Andrea EGGER Eva-Maria PRIBIL-HAMBERGER and Wim J.A.G. DICTUS (Utrecht, The Netherlands) (sponsored by Roman EISENHUT (NHM Wien) AMS). Christine EXNER Emanuel REDL Angelika GRÜNDLER Alexander REISCHÜTZ AMMER CHAEFER Mag. Sabine H Kurt S Claudia HANDL Denise SCHNEIDER Matthias HARZHAUSER (NHM Wien) Elisabeth SINGER Molluscan Conservation & Biodiversity Franz HOCHSTÖGER Mariti STEINER Organised by Ian KILLEEN (Felixtowe, UK) and Mary SEDDON Christoph HÖRWEG Michael URBANEK (Cardiff, UK) (sponsored by UM). -

Excavations at Blombos Cave and the Blombosfontein Nature Reserve
HOLOCENE PREHISTORY OF THE SOUTHERN CAPE, SOUTH AFRICA: EXCAVATIONS AT BLOMBOS CAVE AND THE BLOMBOSFONTEIN NATURE RESERVE Christopher Stuart Henshilwood Institute for Human Evolution University of the Witwatersrand, Johannesburg, South Africa & Institute for Archaeology, History, Culture and Religion University of Bergen, Bergen, Norway 1 PREFACE During 1992/3 nine Later Stone Age (LSA) coastal midden sites ranging in age from 6960 B.P. to 290 B.P., and representing 28 depositional units were excavated in the Blombosfontein Nature Reserve and in the directly adjacent Blombos Estates, situated 20 km to the west of Still Bay, southern Cape, South Africa (Figs. 1.1). This research formed the core of the authors doctoral thesis ‘Holocene archaeology of the coastal Garcia State Forest, southern Cape, South Africa’ completed at Cambridge University in 1995 (Henshilwood 1995). This monograph is based on the results derived from this research. Additional data derived from the 1997 – 1999 excavations of the Later Stone Age levels at Blombos Cave (BBC) has been added into the text (also see appendix) and a brief review of the results from the 1997 – 2005 excavations of the Middle Stone Age (MSA) levels is included. In this monograph the term Blombosfontein is used to cover both the Blombosfontein Nature Reserve and the Blombos Estates. On some older topographical maps the area is designated Garcia State Forest but the currently accepted name is Blombosfontein (lit. flower bush spring). The sites excavated were originally numbered from 1 – 9 and given the acronym GSF (Henshilwood 1995). In this monograph the acronym has been changed to BBF (for Blombosfontein); BBF 1 is the oldest and BBF 9 the youngest site (Fig. -

Association of Juveniles of Four Fish Species With
ASSOCIATION OF JUVENILES OF FOUR FISH SPECIES WITH SANDBANKS IN DURBAN BAY, KWAZULU NATAL M. A. Graham Submitted in partial fulfilment of the requirements for the degree of Master of Science in the Department of Biology at the University of Natal Durban 1994 PREFACE The work described in this thesis was carried out in the Department of Biology, University of Natal, Durban from January 1993 to December 1994, under the supervision of Prof. A. T, Forbes. It was co-supervised by Professor D.P. Cyrus, Department of Zoology, University of Zululand. These stu,dies represent original work by the author and have not been submitted in any form to another university. Where use was made of the work of others it has been duly acknowledged in the text. 11 ACKNOWLEDGEMENTS I would like to express my sincere appreciation to my supervisors, Professor Ticky Forhes and Professor Digby Cyrus, for their support, assistance and guidance throughout this study. Bryan, thanks for your patience, support and encouragment especially while I was writing up this manuscript, for proof-reading and commenting on the drafts. Thanks to Nicky Demetriades for her advice and encouragement. Particular thanks are extended to Kevin Weerts, Dr Alan Connell (CSIR) and Mr Heath Thorpe (CSIR) for confinning identification of benthic organisms. Kevin is also gratefully acknowledged for assistance with statistical analyses. Thank you also to all the willing volunteers who assisted with the collection of samples. The FRD is acknowledged and thanked for financial assistance provided for the duration of the study. Finally, I am especially grateful to my parents who supported and encouraged me throughout my studies. -
Checklist of the Fresh and Brackish Water Snails
Checklist of the fresh and brackish water snails (Mollusca, Gastropoda) of Bénin and adjacent West African ecoregions Zinsou Cosme Koudenoukpo, Olaniran Hamed Odountan, Bert van Bocxlaer, Rose Sablon, Antoine Chikou, Thierry Backeljau To cite this version: Zinsou Cosme Koudenoukpo, Olaniran Hamed Odountan, Bert van Bocxlaer, Rose Sablon, Antoine Chikou, et al.. Checklist of the fresh and brackish water snails (Mollusca, Gastropoda) of Bénin and adjacent West African ecoregions. Zookeys, Pensoft, 2020, 942, pp.21-64. 10.3897/zookeys.942.52722. hal-02917178 HAL Id: hal-02917178 https://hal.archives-ouvertes.fr/hal-02917178 Submitted on 18 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ZooKeys 942: 21–64 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.942.52722 CHECKLIST https://zookeys.pensoft.net Launched to accelerate biodiversity research Checklist of the fresh and brackish water snails (Mollusca, Gastropoda) of Bénin and adjacent West African ecoregions Zinsou Cosme Koudenoukpo1,2*, Olaniran Hamed Odountan3,4,5*, Bert -
Catalog of Recent Molluscan Types in the Natural History Museum of Los
CATALOG OF RECENT MOLLUSCAN TYPES IN THE NATURAL HISTORY MUSEUM OF LOS ANGELES COUNTY compiled by Lindsey T. Groves NATURAL HISTORY MUSEUM OF LOS ANGELES COUNTY MALACOLOGY SECTION 900 Exposition Boulevard, Los Angeles, California, 90007, USA [email protected] [last updated 3 January, 2012] Abstract. Recent molluscan types currently housed in the Malacology Section of the Natural History Museum of Los Angeles County are listed herein. Each type lot entry includes original species/subspecies name, original genus, author, original citation, type status, LACM number, number of specimens and preservation, type locality or collection locality, collector [if known], date collected [if known], and current taxonomic status if different from original description. INTRODUCTION This catalog is published in accordance with Recommendation 72F.4 of the International Code of Zoological Nomenclature (Ride & others, 2000:79). This listing includes all molluscan type material currently housed at the Natural History Museum of Los Angeles County and supercedes the type listing of Sphon (1971). To date 1922 molluscan type lots (71,000+ specimens) are reported for 1147 species and 99 subspecies, which includes 589 holotypes, 1277 paratype lots, 10 lectotypes, 13 paralectotype lots, 29 syntype lots, 3 neotypes, and 2 neotype lots in 550+ references. Type categories listed include: Holotype (in red), paratype(s) (in blue), syntype(s) (in orange), lectotype (in pink), paralectotype(s) (in aqua), and neotype and/or neotype lots (in brown) as defined by Ride & others (2000). Because the term cotype is not recognized by the ICZN, lots originally designated as cotype(s) (in green) by original specimen labels are now referred to as syntype(s). -

Liste De Référence Annotée Des Mollusques Continentaux De France Annotated Checklist of the Continental Molluscs from France
MalaCo (2011) 7, 307-382 Liste de référence annotée des mollusques continentaux de France Annotated checklist of the continental molluscs from France 1 2 3 4 5 Olivier GARGOMINY , Vincent PRIE , Jean-Michel BICHAIN , Xavier CUCHERAT , Benoît FONTAINE 1 Service du Patrimoine Naturel, Muséum national d’Histoire naturelle, CP41, 36 rue Geoffroy Saint-Hilaire, 75005 Paris 2 Biotope, Service R&D, 22, Bd Maréchal Foch, BP58, 34140 Mèze 3 1, rue des Forgerons, 68140 Gunsbach 4 Biotope, Nord-Littoral, Avenue de l’Europe/ZA de la Maie, 62720 Rinxent 5 CERSP UMR 7204, Département Ecologie et Gestion de la Biodiversité, Muséum national d’Histoire naturelle, CP 51 55 rue Buffon, 75005 Paris Correspondance : [email protected] Résumé – Depuis la publication de la Liste de référence des mollusques continentaux de France par Falkner et al. (2002), le nombre de changements est tel qu’une mise à jour a été nécessaire. Nous présentons donc une Liste de référence réactualisée, où tous les changements (nouveaux noms, actes taxonomiques et nomenclaturaux) par rapport à celle de 2002 sont liés à une note faisant référence à la bibliographie concernée, consultée jusqu’à fin 2010. Au total, ce sont 93 taxons terminaux qui viennent enrichir la malacofaune de France, dont 61 récemment décrits et 20 qui en sont retirés. Cette faune compte actuellement 783 taxons terminaux (695 espèces) avec un taux d’endémisme de 43 %. La présente liste a pour vocation de fournir le nouveau cadre taxonomique résultant de dix années de recherches sur la systématique des mollusques continentaux. Aucun acte nomenclatural ou taxonomique nouveaux n’a été réalisé lors de ce travail.