The Significance of Shore Height in Intertidal Macrobenthic Seagrass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download (8MB)
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] ASPECTS OF THE BIOLOGY OF THE SQUAT LOBSTER, MUNIDA RUGOSA (FABRICIUS, 1775). Khadija Abdulla Yousuf Zainal, BSc. (Cairo). A thesis submitted for the degree of Doctor of Philosophy to the Faculty of Science at the University of Glasgow. August 1990 Department of Zoology, University of Glasgow, Glasgow, G12 8QQ. University Marine Biological Station, Millport, Isle of Cumbrae, Scotland KA28 OEG. ProQuest Number: 11007559 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11007559 Published by ProQuest LLC(2018). -

Molluscan Studies
Journal of The Malacological Society of London Molluscan Studies Journal of Molluscan Studies (2014) 80: 412–419. doi:10.1093/mollus/eyu037 Advance Access publication date: 20 June 2014 Morphological expression and histological analysis of imposex in Gemophos viverratus (Kiener, 1834) (Gastropoda: Buccinidae): a new bioindicator of tributyltin pollution on the West African coast Ruy M. A. Lopes-dos-Santos1, Corrine Almeida2, Maria de Lourdes Pereira3, Carlos M. Barroso4 and Susana Galante-Oliveira4 1Biology Department, University of Aveiro, Campus Universita´rio de Santiago, 3810-193 Aveiro, Portugal; 2Department of Engineering and Sea Sciences (DECM), University of Cabo Verde, Campus da Ribeira de Julia˜o, CP 163 Sa˜o Vicente, Cabo Verde; 3Biology Department & CICECO, University of Aveiro, Campus Universita´rio de Santiago, 3810-193 Aveiro, Portugal; and 4Biology Department & CESAM, University of Aveiro, Campus Universita´rio de Santiago, 3810-193 Aveiro, Portugal Correspondence: R. M. A. Lopes-dos-Santos; e-mail: [email protected] (Received 8 January 2014; accepted 24 March 2014) ABSTRACT We describe the reproductive system and morphological expression of imposex in the marine gastropod Gemophos viverratus (Kiener, 1834) for the first time. This species can be used as a bioindicator of tributyl- tin (TBT) pollution on the west coast of Africa, a region for which there is a lack of information regard- ing levels and impacts of TBT pollution. Adult G. viverratus were collected between August 2012 and July 2013 in Sa˜o Vicente Island (Republic of Cabo Verde), at pristine sites on the northeastern and eastern coasts and at Porto Grande Bay, the major harbour in the country. -

Upper Eocene) of the Sultanate of Oman
Pala¨ontol Z (2016) 90:63–99 DOI 10.1007/s12542-015-0277-1 RESEARCH PAPER Terrestrial and lacustrine gastropods from the Priabonian (upper Eocene) of the Sultanate of Oman 1 1 2 3 Mathias Harzhauser • Thomas A. Neubauer • Dietrich Kadolsky • Martin Pickford • Hartmut Nordsieck4 Received: 17 January 2015 / Accepted: 15 September 2015 / Published online: 29 October 2015 Ó The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Terrestrial and aquatic gastropods from the sparse non-marine fossil record of the Eocene in the Tethys upper Eocene (Priabonian) Zalumah Formation in the region. The occurrence of the genera Lanistes, Pila, and Salalah region of the Sultanate of Oman are described. The Gulella along with some pomatiids, probably related to assemblages reflect the composition of the continental extant genera, suggests that the modern African–Arabian mollusc fauna of the Palaeogene of Arabia, which, at that continental faunas can be partly traced back to Eocene time, formed parts of the southeastern Tethys coast. Sev- times and reflect very old autochthonous developments. In eral similarities with European faunas are observed at the contrast, the diverse Vidaliellidae went extinct, and the family level, but are rarer at the genus level. These simi- morphologically comparable Neogene Achatinidae may larities point to an Eocene (Priabonian) rather than to a have occupied the equivalent niches in extant environ- Rupelian age, although the latter correlation cannot be ments. Carnevalea Harzhauser and Neubauer nov. gen., entirely excluded. At the species level, the Omani assem- Arabiella Kadolsky, Harzhauser and Neubauer nov. gen., blages lack any relations to coeval faunas. -

Two New Paleogene Species of Mud Shrimp (Crustacea, Decapoda, Upogebiidae) from Europe and North America
Bulletin of the Mizunami Fossil Museum, no. 33 (2006), p. 77–85, 2 pls., 1 fig. © 2006, Mizunami Fossil Museum Two new Paleogene species of mud shrimp (Crustacea, Decapoda, Upogebiidae) from Europe and North America Rene H. B. Fraaije1, Barry W. M. van Bakel1, John W. M. Jagt2, and Yvonne Coole3 1Oertijdmuseum De Groene Poort, Bosscheweg 80, NL-5283 WB Boxtel, the Netherlands <[email protected]> 2Natuurhistorisch Museum Maastricht, de Bosquetplein 6-7, NL-6211 KJ Maastricht, the Netherlands <[email protected]> 3St. Maartenslaan 88, NL-6039 BM Stramproy, the Netherlands Abstract Two new species of the mud shrimp genus Upogebia (Callianassoidea, Upogebiidae) are described; U. lambrechtsi sp. nov. from the lower Eocene (Ypresian) of Egem (northwest Belgium), and U. barti sp. nov. from the upper Oligocene (Chattian) of Washington State (USA). Both new species here described have been collected from small, ball-shaped nodules; they are relatively well preserved and add important new data on the palaeobiogeographic distribution of fossil upogebiids. Key words: Crustacea, Decapoda, Upogebiidae, Eocene, Oligocene, Belgium, USA, new species Introduction Jurassic species of Upogebia have been recorded, in stratigraphic order: On modern tidal flats, burrowing upogebiid shrimps constitute the dominant decapod crustacean group. For instance, in the 1 – Upogebia rhacheochir Stenzel, 1945 (p. 432, text-fig. 12; pl. intertidal zone of the northern Adriatic (Mediterranean, southern 42); Britton Formation (Eagle Ford Group), northwest of Dallas Europe) up to 200 individuals per square metre have been recorded (Texas, USA). Stenzel (1945, p. 408) dated the Britton (Dworschak, 1987). Worldwide, several dozens of species of Formation as early Turonian, but a late Cenomanian age is Upogebia and related genera are known, and their number is still more likely (compare Jacobs et al., 2005). -

Biology of Mediterranean and Caribbean Thalassinidea (Decapoda)
Biology of Mediterranean and Caribbean Thalassinidea (Decapoda) Peter C. DWORSCHAK Dritte Zoologische Abteilung, Naturhistorisches Museum, Burgring 7, A 1014 Wien, Austria ([email protected]) Abstract Among burrowing organisms, the most complex and extensive burrow systems are found within the Thalassinidea. This group of decapods comprises some 520+ species in currently 11 families and 80+ genera. They live predominantly in very shallow waters, where they often occur in high densities and influence the whole sedimentology and geochemistry of the seabed. In this contribution I present results of studies on the biology of several Mediterranean (Upogebia pusilla, U. tipica, Pestarella tyrrhena, P. candida, Jaxea nocturna) and Caribbean (Axiopsis serratifrons, Neocallichirus grandimana, Glypturus acanthochirus, Corallianassa longiventris) species. Of special interest is the occurrence of debris-filled chambers in the burrows of two callianassid species, Pestarella tyrrhena and Corallianassa longiventris. The possible role of this introduced plant material for the nutrition of the shrimps is discussed. 1. Introduction The Thalassinidea are a group of mainly burrowing decapod shrimps. They have attracted increased attention in recent ecological studies on marine soft-bottom benthos, especially in terms of their influence on the whole sedimentology and geochemistry of the seabed (Ziebis et al., 1996a, b), their bioturbating activities (Rowden & Jones, 1993; Rowden et al., 1998a, b) and consequent effects on benthic community structure (Posey, 1986; Posey et al., 1991; Wynberg & Branch, 1994; Tamaki, 1994). They are of particular interest because they construct very complex and extensive burrow systems (Griffis & Suchanek, 1991; Nickell & Atkinson, 1995). This group of decapods comprises some 520+ species in currently 11 families and 80+ genera. -

Determination of the Biologically Relevant Sampling Depth for Terrestrial and Aquatic Ecological Risk Assessments
EPA/600/R-15/176 ERASC-015F October 2015 DETERMINATION OF THE BIOLOGICALLY RELEVANT SAMPLING DEPTH FOR TERRESTRIAL AND AQUATIC ECOLOGICAL RISK ASSESSMENTS Ecological Risk Assessment Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH NOTICE This document has been subjected to the Agency’s peer and administrative review and has been approved for publication as an EPA document. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Cover art on left-hand side is an adaptation of illustrations in two Soil Quality Information Sheets published by the USDA, Natural Resources Conservation Service in May 2001: 1) Rangeland Sheet 6, Rangeland Soil Quality—Organic Matter, and 2) Rangeland Sheet 8, Rangeland Soil Quality—Soil Biota. Cover art on right-hand side is an adaptation of an illustration from Life in the Chesapeake Bay, by Alice Jane Lippson and Robert L. Lippson, published by Johns Hopkins University Press, 2715 North Charles Street, Baltimore, MD 21218. Preferred Citation: U.S. EPA (U.S. Environmental Protection Agency). 2015. Determination of the Biologically Relevant Sampling Depth for Terrestrial and Aquatic Ecological Risk Assessments. National Center for Environmental Assessment, Ecological Risk Assessment Support Center, Cincinnati, OH. EPA/600/R-15/176. ii TABLE OF CONTENTS LIST OF TABLES ........................................................................................................................ -

Variability in Middle Stone Age Symbolic Traditions: the Marine Shell Beads from Sibudu Cave, South Africa Marian Vanhaeren, Lyn Wadley, Francesco D’Errico
Variability in Middle Stone Age symbolic traditions: The marine shell beads from Sibudu Cave, South Africa Marian Vanhaeren, Lyn Wadley, Francesco D’errico To cite this version: Marian Vanhaeren, Lyn Wadley, Francesco D’errico. Variability in Middle Stone Age symbolic tra- ditions: The marine shell beads from Sibudu Cave, South Africa. Journal of Archaeological Science: Reports, Elsevier, 2019, 27, pp.101893. 10.1016/j.jasrep.2019.101893. hal-02998635 HAL Id: hal-02998635 https://hal.archives-ouvertes.fr/hal-02998635 Submitted on 11 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Manuscript Details Manuscript number JASREP_2017_485_R1 Title Variability in Middle Stone Age symbolic traditions: the marine shell beads from Sibudu Cave, South Africa Short title Marine shell beads from Sibudu Article type Research Paper Abstract Located in the KwaZulu-Natal, 15 km from the coast, Sibudu has yielded twenty-three marine gastropods, nine of which perforated. At 70.5 ± 2.0 ka, in a Still Bay Industry, there is a cluster of perforated Afrolittorina africana shells, one of which has red ochre stains. There is also a perforated Mancinella capensis and some unperforated shells of both A. -
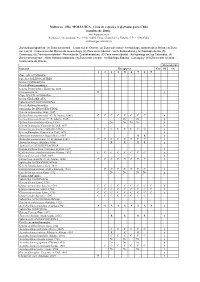
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Methods Collecting Axiidea and Gebiidea (Decapoda): a Review 5-21 Ann
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Annalen des Naturhistorischen Museums in Wien Jahr/Year: 2015 Band/Volume: 117B Autor(en)/Author(s): Dworschak Peter C. Artikel/Article: Methods collecting Axiidea and Gebiidea (Decapoda): a review 5-21 Ann. Naturhist. Mus. Wien, B 117 5–21 Wien, Jänner 2015 Methods collecting Axiidea and Gebiidea (Decapoda): a review P.C. Dworschak* Abstract Axiidea and Gebiidae (formerly treated together as Thalassinidea) have a crypic lifestyle. Collecting these shrimp therefore requires special field methods. The present paper reviews these methods according to habitats and provides recommendations as well as data on their efficiency. In addition, information on the preservation of these animals is presented. Key words: Thalassinidea, Axiidea, Gebiidea, method, collecting, preservation Zusammenfassung Maulwurfskrebse aus den zwei Unterordungen der zehnfüßigen Krebes Axiidea und Gebiidea (früher zusammengefaßt als Thalassinidea) kommen in temperaten, subtropischen und tropischen Meeren vor und zeichnen sich durch ein Leben im Verborgenen aus. Viele Arten legen tiefe und ausgedehnte Bauten an. Diese Lebensweise erfordert eigene Methoden, um die Krebse zu fangen. Die verschiedenen Fangmethoden werden hier vorgestellt und Angaben zur Effizienz gemacht. Zusätzlich werden Angaben zur Fixierung und Konservierung der Krebse präsentiert. Introduction Formerly treated together as the thalassinideans, the infraorders Gebiidea DE SAINT LAURENT, 1979 and Axiidea DE SAINT LAURENT, 1979 represent two distinctly separate groups of decapods (ROBLES et al. 2009; BRACKEN et al. 2009; DWORSCHAK et al. 2012, POORE et al., 2014). They are commonly called mud shrimp or ghost shrimp, although they are only distantly related to true (dendrobranchiate or caridean) shrimp. -

THREATENED and ENDANGERED SPECIES of NEW MEXICO 2008 Biennial Review and Recommendations
THREATENED AND ENDANGERED SPECIES OF NEW MEXICO 2008 BIENNIAL REVIEW DRAFT First Public Comment Period March 11, 2008 New Mexico Department of Game and Fish Conservation Services Division DRAFT 2008 Biennial Review of T & E Species of NM, 3/11/08 THREATENED AND ENDANGERED SPECIES OF NEW MEXICO 2008 Biennial Review and Recommendations Authority: Wildlife Conservation Act (17-2-37 through 17-2-46 NMSA 1978) EXECUTIVE SUMMARY: A total of 118 species and subspecies are on the 2008 list of threatened and endangered New Mexico wildlife. The list includes 2 crustaceans, 25 mollusks, 23 fishes, 6 amphibians, 15 reptiles, 32 birds and 15 mammals (Tables 1, 2). An additional 7 species of mammals has been listed as restricted to facilitate control of traffic in federally protected species. A species is endangered if it is in jeopardy of extinction or extirpation from the state; a species is threatened if it is likely to become endangered within the foreseeable future throughout all or a significant portion of its range in New Mexico. Species or subspecies of mammals, birds, reptiles, amphibians, fishes, mollusks, and crustaceans native to New Mexico may be listed as threatened or endangered under the Wildlife Conservation Act (WCA). During the Biennial Review, species may be upgraded from threatened to endangered, or downgraded from endangered to threatened, based upon data, views, and information regarding the biological and ecological status of the species. Investigations for new listings or removals from the list (delistings) can be undertaken at any time, but require additional procedures from those for the Biennial Review. The 2006 Biennial Review contained a recommendation for maintaining the status for 119 species and subspecies listed as threatened, endangered, or restricted under the WCA, and uplisting four species (Arizona grasshopper sparrow, Pecos bluntnose shiner, spikedace, and meadow jumping mouse ) from threatened to endangered and downlisting two species (shortneck snaggletooth and piping plover) from endangered to threatened. -

Currently Upogebia Capensis; Crustacea, Decapoda): Proposed Replacement of Neotype, So Conserving the Usage of Capensis and Also That of C
Bulletin of Zoological Nomenclature 49(3) September 1992 Case 2827 Gebia major capensis Kraiiss, 1843 (currently Upogebia capensis; Crustacea, Decapoda): proposed replacement of neotype, so conserving the usage of capensis and also that of C. africana Ortmann, 1894 (currently Upogebia africana) N. Ngoc-Ho Laboratoire de Zoologie (Arthropodes), Museum National d'Histoire Naturelle, 61 rue de Buffon, 75231 Paris, France Gary C.B. Poore Department of Crustacea, Museum of Victoria, Swanston Street, Melbourne, Victoria 3000, Australia Abstract. The purpose of this application is to conserve the accustomed usage of the specific names of two South African species of prawns: Upogebia capensis (Krauss, 1843) and U. africana (Ortmann, 1894). The latter species is commonly known as the mud-prawn or mud-shrimp. It is proposed to designate a replacement neotype for capensis from material of the species as presently understood; the previously designated neotype is a specimen oi africana. 1. Three species oi Gebia Leach, 1815 (p. 342; family UPOGEBIIDAE) were described from South Africa. Gebia major var. capensis Krauss, 1843 (p. 54) was originally described as a variety of Gebia major de Haan, [1841] (pi. 35, fig. 7; text (p. 165) published in [1849]; see Sherborn & Jentink (1895, p. 150) and Holthuis (1953, p. 37) for the dates of publication). The type material from Table Bay is now lost. The original description was short and by modern standards very incomplete and cannot be definitely reconciled with any single species known today. G. subspinosa Stimpson, 1860 (p. 22) was described from Simon's Bay; the fate of its type material is unknown. -

Liste De Référence Annotée Des Mollusques Continentaux De France Annotated Checklist of the Continental Molluscs from France
MalaCo Le journal de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Strasbourg / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIÉ / Montpellier / [email protected] Pour soumettre un article à MalaCo : 1ère étape – Le premier auteur veillera à ce que le manuscrit soit conforme aux recommandations aux auteurs (consultez le site www.journal-malaco.fr). Dans le cas contraire, la rédaction peut se réserver le droit de refuser l’article. 2ème étape – Joindre une lettre à l’éditeur, en document texte, en suivant le modèle suivant : "Veuillez trouvez en pièce jointe l’article rédigé par << mettre les noms et prénoms de tous les auteurs>> et intitulé : << mettre le titre en français et en anglais >> (avec X pages, X figures et X tableaux). Les auteurs cèdent au journal MalaCo (ISSN1778-3941) le droit de publication de ce manuscrit et ils garantissent que l’article est original, qu’il n’a pas été soumis pour publication à un autre journal, n’a pas été publié auparavant et que tous sont en accord avec le contenu." 3ème étape – Envoyez par voie électronique le manuscrit complet (texte et figures) en format .doc et la lettre à l’éditeur à : [email protected]. Pour les manuscrits volumineux (>5 Mo), envoyez un courriel à la même adresse pour élaborer une procédure FTP pour le dépôt du dossier final.