Association of Juveniles of Four Fish Species With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upper Eocene) of the Sultanate of Oman
Pala¨ontol Z (2016) 90:63–99 DOI 10.1007/s12542-015-0277-1 RESEARCH PAPER Terrestrial and lacustrine gastropods from the Priabonian (upper Eocene) of the Sultanate of Oman 1 1 2 3 Mathias Harzhauser • Thomas A. Neubauer • Dietrich Kadolsky • Martin Pickford • Hartmut Nordsieck4 Received: 17 January 2015 / Accepted: 15 September 2015 / Published online: 29 October 2015 Ó The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Terrestrial and aquatic gastropods from the sparse non-marine fossil record of the Eocene in the Tethys upper Eocene (Priabonian) Zalumah Formation in the region. The occurrence of the genera Lanistes, Pila, and Salalah region of the Sultanate of Oman are described. The Gulella along with some pomatiids, probably related to assemblages reflect the composition of the continental extant genera, suggests that the modern African–Arabian mollusc fauna of the Palaeogene of Arabia, which, at that continental faunas can be partly traced back to Eocene time, formed parts of the southeastern Tethys coast. Sev- times and reflect very old autochthonous developments. In eral similarities with European faunas are observed at the contrast, the diverse Vidaliellidae went extinct, and the family level, but are rarer at the genus level. These simi- morphologically comparable Neogene Achatinidae may larities point to an Eocene (Priabonian) rather than to a have occupied the equivalent niches in extant environ- Rupelian age, although the latter correlation cannot be ments. Carnevalea Harzhauser and Neubauer nov. gen., entirely excluded. At the species level, the Omani assem- Arabiella Kadolsky, Harzhauser and Neubauer nov. gen., blages lack any relations to coeval faunas. -

Estuarine Fish Diversity of Tamil Nadu, India
Indian Journal of Geo Marine Sciences Vol. 46 (10), October 2017, pp. 1968-1985 Estuarine fish diversity of Tamil Nadu, India H.S. Mogalekar*, J. Canciyal#, P. Jawahar, D.S. Patadiya, C. Sudhan, P. Pavinkumar, Prateek, S. Santhoshkumar & A. Subburaj Department of Fisheries Biology and Resource Management, Fisheries College & Research Institute, (Tamil Nadu Fisheries University), Thoothukudi-628 008, India. #ICAR-National Academy of Agricultural Research Management, Rajendranagar, Hyderabad-500 030, Telangana, India. *[E-Mail: [email protected]] Received 04 February 2016 ; revised 10 August 2017 Systematic and updated checklist of estuarine fishes contains 330 species distributed under 205 genera, 95 families, 23 orders and two classes. The most diverse order was perciformes with 175 species, 100 genera and 43 families. The top four families with the highest number of species were gobidae (28 species), carangidae (23 species), engraulidae (15 species) and lutjanidae (14 species). Conservation status of all taxa includes one species as endangered, five species as vulnerable, 14 near threatened, 93 least concern and 16 data deficient. As numbers of commercial, sports, ornamental and cultivable fishes are high, commercial and recreational fishing could be organized. Seed production by selective breeding is recommended for aquaculture practices in estuarine areas of Tamil Nadu. [Keywords: Estuarine fishes, updated checklist, fishery and conservation status, Tamil Nadu] Introduction significant component of coastal ecosystem due to The total estuarine area of Tamil Nadu their immense biodiversity values in aquatic was estimated to be 56000 ha, which accounts ecology. The fish fauna inhabiting the estuarine 3.88 % of the total estuarine area of India 1. -

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

A Retrospective Analysis of Shark Catches Made by Pelagic Longliners
OCEANOGRAPHIC RESEARCH INSTITUTE, DURBAN, SOUTH AFRICA, AFFILIATED WITH THE UNIVERSITY OF KWAZULU-NATAL A retrospective analysis of shark catches made by pelagic longliners off the east coast of South Africa and biology and life history of shortfin mako shark, Isurus oxyrinchus Submitted in fulfillment of the academic requirements for the degree of Master of Science in the Oceanographic Research Institute, affiliated with the School of Biological and Conservation Sciences, University of KwaZulu-Natal, Durban. By Alan Foulis February 2013 i i. Abstract Oceanic pelagic shark species are under threat worldwide as fishing effort increases and they are taken as both targeted and bycatch. It is widely recognized that the life history characteristics of sharks make them inherently susceptible to over- exploitation and as a result many shark-directed fisheries have collapsed. It is therefore essential that good-quality data are collected and analyzed in order to provide fisheries managers with the right information to manage these species sustainably. South Africa has a pelagic longline fishery which includes tuna-, swordfish-, and shark-directed vessels. This study analyzed logbook (1998 – 2010) and observer data (2002 – 2010) provided by the Department of Agriculture, Forestry and Fisheries in order to assess the catch composition and standardized catch-per-unit-effort (CPUE) of sharks captured as both targeted catch and bycatch. The study area consisted of four zones moving east of the 20°E meridian: the Agulhas Bank (20°E – 24°E), South Coast (25°E – 29°E), East Coast 1 (30°E – 32.8°E), and East Coast 2 (32.9°E – 36.5°E). -

Rapport (2.454Mb)
NINA28 Report 55 Movements and area use by small spotted grunter (Pomadasys commersonnii) in the Great Fish Estuary (South Africa): implication for management T.F. Næsje, A.R. Childs, P.D. Cowley, E.B. Thorstad, F. Økland, S. Weerts and P. Buthelezi Øvre venstre hjørne på konvolutt Øvre venstre hjørne på konvolutt Øvre venstre hjørne på konvolutt E6/5 T EAMWORK E NTHUSIASM I NTEGRITY Q UALITY Cooperation and expertise for a sustainable future . Movements and area use by small spotted grunter (Pomadasys commersonnii) in the Great Fish Estuary (South Africa): implication for management T.F. Næsje1, A.R. Childs2, P.D. Cowley3, E.B. Thorstad1, F. Økland1, S. Weerts4 and P. Buthelezi4 1 Norwegian Institute for Nature Research (NINA), Tungasletta 2, NO-7485 Trondheim, Norway 2 Department of Ichthyology and Fisheries Science (DIFS), Rhodes University, P.O. Box 94, Grahamstown 6140, South Africa 3 South African Institute for Aquatic Biodiversity (SAIAB), Private Bag 1015, Grahamstown, South Africa 4 Zoology Department, University of Zululand, Private Bag X1001, KwaDlangezwa 3886, South Africa NINA Report 55 Næsje, T.F., Childs, A.R., Cowley, P.D., Thorstad, E.B., Økland, F., Weerts, S., and Buthelezi, P. 2005. Movements and area use by small spotted grunter (Pomadasys commersonnii) in the Great Fish Estuary (South Africa): implication for management. - NINA Report 55. 46 pp. Trondheim, May 2005 ISSN: 1504-3312 ISBN: 82-426-1588-8 (pdf) COPYRIGHT © Norwegian Institute for Nature Reseach (NINA) and South African Institute for Aquatic Biodiversity (SAIAB). The publication may be freely cited where the source is acknowledged. AVAILABILITY Open QUALITY CONTROLLED BY Odd Terje Sandlund, NINA RESPONSIBLE SIGNATURE Research director Odd Terje Sandlund (sign.) CLIENT(S) The South Africa / Norway Programme on Research Collaboration (National Research Foundation of South Africa, and Research Council of Norway) PHOTOES T. -

The Significance of Shore Height in Intertidal Macrobenthic Seagrass
AQUATIC CONSERVATION: MARINE AND FRESHWATER ECOSYSTEMS Aquatic Conserv: Mar. Freshw. Ecosyst. 21: 614–624 (2011) Published online 20 October 2011 in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/aqc.1234 The significance of shore height in intertidal macrobenthic seagrass ecology and conservation R. S. K. BARNESa,b,* and M. D. F. ELLWOODb aKnysna Field Laboratory, Department of Zoology and Entomology, Rhodes University, P.O. Box 1186, Knysna, RSA bDepartment of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK ABSTRACT 1. Benthic faunal assemblages of an intertidal seagrass bed were sampled at three shore heights (LWN, MLW, LWS) at the mouth, mid-point and head of the Steenbok Channel in South Africa’s premier seagrass site, the warm-temperate Knysna estuarine bay, Garden Route National Park. 2. Faunal abundance, species richness, species diversity, and proportion of rare species were relatively uniform along the Channel, as were faunal abundance, species diversity and proportion of rare species down the shore. Overall species richness per station, however, was significantly lower at LWN than at either MLW or LWS, although the distribution of species richness over the shore did not depart from random at one of the three sites. Overall faunal abundance and those of individual component species were dispersed patchily through the bed. 3. The nature of the faunal assemblages present, however, varied significantly throughout the bed, both along the Channel at each shore-height horizon, and down the shore at each site. LWN assemblages formed a unit distinct from those at MLW and LWS. Overall, the shore-height axis accounted for 47% and the along-shore axis 28% of total assemblage variation. -
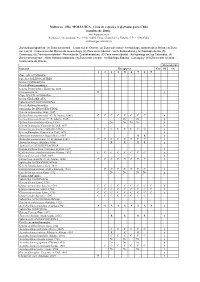
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

MATURATION and SPAWNING of the SMALLSPOTTED GRUNT Pomadasys Commersonnii (LACEPÈDE, 1801) in the ARABIAN SEA OFF OMAN
Thalassas, 27 (1): 67-79 An International Journal of Marine Sciences MATURATION AND SPAWNING OF THE SMALLSPOTTED GRUNT Pomadasys commersonnii (LACEPÈDE, 1801) IN THE ARABIAN SEA OFF OMAN A. AL-NAHDI, A. AL-MARZOUQI, N. JAYABALAN(1) & S. AL-HABSI Key words: Pomadasys commersonnii, Lm, GSI, relative condition, spawning, fecundity, sex-ratio, Arabian Sea, Oman ABSTRACT INTRODUCTION Observations on the maturation and spawning Fishes of the genus Pomadasys (Family: of the smallspotted grunt Pomadasys commersonnii Haemulidae) are represented by 11 species in caught from the Arabian Sea off Oman were made Omani waters (Fouda et al., 1997). The species between April 2005 and March 2007. While, males P. commersonnii popularly called smallspotted matured (50% maturity) at 44.2 cm TL, females grunt is one of the commonest fishes caught by matured at 42 cm TL. The population of P. the artisanal and industrial trawlers from Omani commersonnii spawned during June-December. The waters. The species is distributed along the northwest monthly gonado-somatic indices and the occurrence coast of India to the southern Oman, in the south of spent gonads coincided with the peak spawning to Seychelles, Madagascar and South Africa in the season. However, the monthly relative condition was coastal waters, estuaries, tidal fissures and even can not indicative of the spawning activity. Individual fish tolerate fresh water (Smith and Heemstra, 1986). spawned more than once a year. Fecundity ranged Besides forming a good recreational fishery (Smith from 214,510 to 1,421,520 eggs. Sex-ratio indicated and McKay, 1986; Mann et al., 2002), the species has homogeneous distribution of males and females in also been identified as a potent candidate for intensive the population. -

THREATENED and ENDANGERED SPECIES of NEW MEXICO 2008 Biennial Review and Recommendations
THREATENED AND ENDANGERED SPECIES OF NEW MEXICO 2008 BIENNIAL REVIEW DRAFT First Public Comment Period March 11, 2008 New Mexico Department of Game and Fish Conservation Services Division DRAFT 2008 Biennial Review of T & E Species of NM, 3/11/08 THREATENED AND ENDANGERED SPECIES OF NEW MEXICO 2008 Biennial Review and Recommendations Authority: Wildlife Conservation Act (17-2-37 through 17-2-46 NMSA 1978) EXECUTIVE SUMMARY: A total of 118 species and subspecies are on the 2008 list of threatened and endangered New Mexico wildlife. The list includes 2 crustaceans, 25 mollusks, 23 fishes, 6 amphibians, 15 reptiles, 32 birds and 15 mammals (Tables 1, 2). An additional 7 species of mammals has been listed as restricted to facilitate control of traffic in federally protected species. A species is endangered if it is in jeopardy of extinction or extirpation from the state; a species is threatened if it is likely to become endangered within the foreseeable future throughout all or a significant portion of its range in New Mexico. Species or subspecies of mammals, birds, reptiles, amphibians, fishes, mollusks, and crustaceans native to New Mexico may be listed as threatened or endangered under the Wildlife Conservation Act (WCA). During the Biennial Review, species may be upgraded from threatened to endangered, or downgraded from endangered to threatened, based upon data, views, and information regarding the biological and ecological status of the species. Investigations for new listings or removals from the list (delistings) can be undertaken at any time, but require additional procedures from those for the Biennial Review. The 2006 Biennial Review contained a recommendation for maintaining the status for 119 species and subspecies listed as threatened, endangered, or restricted under the WCA, and uplisting four species (Arizona grasshopper sparrow, Pecos bluntnose shiner, spikedace, and meadow jumping mouse ) from threatened to endangered and downlisting two species (shortneck snaggletooth and piping plover) from endangered to threatened. -

Liste De Référence Annotée Des Mollusques Continentaux De France Annotated Checklist of the Continental Molluscs from France
MalaCo Le journal de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Strasbourg / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIÉ / Montpellier / [email protected] Pour soumettre un article à MalaCo : 1ère étape – Le premier auteur veillera à ce que le manuscrit soit conforme aux recommandations aux auteurs (consultez le site www.journal-malaco.fr). Dans le cas contraire, la rédaction peut se réserver le droit de refuser l’article. 2ème étape – Joindre une lettre à l’éditeur, en document texte, en suivant le modèle suivant : "Veuillez trouvez en pièce jointe l’article rédigé par << mettre les noms et prénoms de tous les auteurs>> et intitulé : << mettre le titre en français et en anglais >> (avec X pages, X figures et X tableaux). Les auteurs cèdent au journal MalaCo (ISSN1778-3941) le droit de publication de ce manuscrit et ils garantissent que l’article est original, qu’il n’a pas été soumis pour publication à un autre journal, n’a pas été publié auparavant et que tous sont en accord avec le contenu." 3ème étape – Envoyez par voie électronique le manuscrit complet (texte et figures) en format .doc et la lettre à l’éditeur à : [email protected]. Pour les manuscrits volumineux (>5 Mo), envoyez un courriel à la même adresse pour élaborer une procédure FTP pour le dépôt du dossier final. -

Marine and Estuarine Fish Fauna of Tamil Nadu, India
Proceedings of the International Academy of Ecology and Environmental Sciences, 2018, 8(4): 231-271 Article Marine and estuarine fish fauna of Tamil Nadu, India 1,2 3 1 1 H.S. Mogalekar , J. Canciyal , D.S. Patadia , C. Sudhan 1Fisheries College and Research Institute, Thoothukudi - 628 008, Tamil Nadu, India 2College of Fisheries, Dholi, Muzaffarpur - 843 121, Bihar, India 3Central Inland Fisheries Research Institute, Barrackpore, Kolkata - 700 120, West Bengal, India E-mail: [email protected] Received 20 June 2018; Accepted 25 July 2018; Published 1 December 2018 Abstract Varied marine and estuarine ecosystems of Tamil Nadu endowed with diverse fish fauna. A total of 1656 fish species under two classes, 40 orders, 191 families and 683 geranra reported from marine and estuarine waters of Tamil Nadu. In the checklist, 1075 fish species were primary marine water and remaining 581 species were diadromus. In total, 128 species were reported under class Elasmobranchii (11 orders, 36 families and 70 genera) and 1528 species under class Actinopterygii (29 orders, 155 families and 613 genera). The top five order with diverse species composition were Perciformes (932 species; 56.29% of the total fauna), Tetraodontiformes (99 species), Pleuronectiforms (77 species), Clupeiformes (72 species) and Scorpaeniformes (69 species). At the family level, the Gobiidae has the greatest number of species (86 species), followed by the Carangidae (65 species), Labridae (64 species) and Serranidae (63 species). Fishery status assessment revealed existence of 1029 species worth for capture fishery, 425 species worth for aquarium fishery, 84 species worth for culture fishery, 242 species worth for sport fishery and 60 species worth for bait fishery. -

Pommadasys Commersonnii (Pisces: Haemulidae)
STUDIES ON CAPTIVE REARING OF SPOTTED GRUNTER, POMMADASYS COMMERSONNII (PISCES: HAEMULIDAE) UNDER AMBIENT CONDITIONS. Submitted in Fulfilment of the . Requirements for the Degree of < MASTER OF SCIENCE of RHODES UNIVERSITY Grahamstown, South Africa by NTOBEKO BACELA April 1998. 11 My father remains my inspiration, sacrificed his family and ultimately, his life ...... 111 TABLE OF CONTENTS ACKNOWLEDGEMENTS ..................................................................................................... v ABSTRACT ............................................................................................................................. vi CHAPTER 1. INTRODUCTION........................................................................................... 1 CHAPTER 2. EXPERIMENTAL LOCATION, SYSTEMS AND GENERAL METHODS. Systems specifications ............................................................................................................ 10 Water quality ............................................................................................................................ 13 General experimental methods ................................................................................................. 13 Monitoring of fish performance ............................................................................................... 15 Statistical methods ................................................................................................................... 18 . CHAPTER 3. THE EFFECT OF STOCKING DENSITY ON THE GROWTH