Near Infrared Photon-Assisted Polymerization of Advanced Polymer Composites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Program of the 76Th Annual Meeting
PROGRAM OF THE 76 TH ANNUAL MEETING March 30−April 3, 2011 Sacramento, California THE ANNUAL MEETING of the Society for American Archaeology provides a forum for the dissemination of knowledge and discussion. The views expressed at the sessions are solely those of the speakers and the Society does not endorse, approve, or censor them. Descriptions of events and titles are those of the organizers, not the Society. Program of the 76th Annual Meeting Published by the Society for American Archaeology 900 Second Street NE, Suite 12 Washington DC 20002-3560 USA Tel: +1 202/789-8200 Fax: +1 202/789-0284 Email: [email protected] WWW: http://www.saa.org Copyright © 2011 Society for American Archaeology. All rights reserved. No part of this publication may be reprinted in any form or by any means without prior permission from the publisher. Program of the 76th Annual Meeting 3 Contents 4................ Awards Presentation & Annual Business Meeting Agenda 5………..….2011 Award Recipients 11.................Maps of the Hyatt Regency Sacramento, Sheraton Grand Sacramento, and the Sacramento Convention Center 17 ................Meeting Organizers, SAA Board of Directors, & SAA Staff 18 ............... General Information . 20. .............. Featured Sessions 22 ............... Summary Schedule 26 ............... A Word about the Sessions 28…………. Student Events 29………..…Sessions At A Glance (NEW!) 37................ Program 169................SAA Awards, Scholarships, & Fellowships 176................ Presidents of SAA . 176................ Annual Meeting Sites 178................ Exhibit Map 179................Exhibitor Directory 190................SAA Committees and Task Forces 194…….…….Index of Participants 4 Program of the 76th Annual Meeting Awards Presentation & Annual Business Meeting APRIL 1, 2011 5 PM Call to Order Call for Approval of Minutes of the 2010 Annual Business Meeting Remarks President Margaret W. -

Fantasy & Science Fiction V030n04
THE MA GAZINE Of Fantasy and JACK VANCE Science Fiction ISAAC ASIMOV J.T. MCINTOS NOVELETS We Can Remember It For You Wholesale Philip k. dick 4 The Sorcerer Pharesm JACK VANCE 79 SHORT STORIES Appoggiatura A. M, MARPLE 25 But Soft, What Light . CAROL EMSHWILLER 41 The Sudden Silence J. T. MCINTOSH 45 The Face Is Familiar GILBERT THOMAS 64 The Space Twins JAMES PULLEY 75 Bordered In Black LARRY NIVEN 112 FEATURES Cartoon GAHAN WILSON 24 Books JUDITH MERRIL 31 Injected Memory THEODORE L. THOMAS 62 Verse: The Octopus DORIS PITKIN BUCK 63 Science: The Nobelmen of Science ISAAC ASIMOV 101 F&SF Marketplace 129 Cover by Jack Gaughan (illustrating "The Sorcerer Pharesm”) Joseph W. Ferman, publishek Edward L. Ferman, editor Ted White, assistant editor Isaac Asimov, science editor Judith Merril, book editor Robert P. Mills, consulting editor Dale Beardale, aRCULATiON manager The Magazine of Fantasy and Science Fiction, Volume 30, No. 4, Whole No. 179, Apr. 1966. Published monthly by Mercury Press, Inc., at 504 o copy. Annual subscription $5.00; $5.50 in Canada and the Pan American Union, $6.00 in all other countries. Publication office, 10 Ferry Street, Concord, N. H. 03302. Editorial and general mail should be sent to 347 East 53rd St., New York, N. Y. 10022. Second Class postage paid at Concord, N. H. Printed in U.S.A. © 1966 by Mercury Press, Inc. All rights including translations into other languages, reserved. Submissions must be accompanied by stamped, self-addressed envelopes: the Publisher assumes no responsibility for return of unsolicited manuscripts. -

The Entomologist's Record and Journal of Variation
M DC, — _ CO ^. E CO iliSNrNVINOSHilWS' S3ldVyan~LIBRARlES*"SMITHS0N!AN~lNSTITUTl0N N' oCO z to Z (/>*Z COZ ^RIES SMITHSONIAN_INSTITUTlON NOIiniIiSNI_NVINOSHllWS S3ldVaan_L: iiiSNi'^NviNOSHiiNS S3iavyan libraries Smithsonian institution N( — > Z r- 2 r" Z 2to LI ^R I ES^'SMITHSONIAN INSTITUTlON'"NOIini!iSNI~NVINOSHilVMS' S3 I b VM 8 11 w </» z z z n g ^^ liiiSNi NviNOSHims S3iyvyan libraries Smithsonian institution N' 2><^ =: to =: t/J t/i </> Z _J Z -I ARIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS SSIdVyan L — — </> — to >'. ± CO uiiSNi NViNosHiiws S3iyvaan libraries Smithsonian institution n CO <fi Z "ZL ~,f. 2 .V ^ oCO 0r Vo^^c>/ - -^^r- - 2 ^ > ^^^^— i ^ > CO z to * z to * z ARIES SMITHSONIAN INSTITUTION NOIinillSNl NVINOSHllWS S3iaVdan L to 2 ^ '^ ^ z "^ O v.- - NiOmst^liS^> Q Z * -J Z I ID DAD I re CH^ITUCnMIAM IMOTtTIITinM / c. — t" — (/) \ Z fj. Nl NVINOSHIIINS S3 I M Vd I 8 H L B R AR I ES, SMITHSONlAN~INSTITUTION NOIlfl :S^SMITHS0NIAN_ INSTITUTION N0liniliSNI__NIVIN0SHillMs'^S3 I 8 VM 8 nf LI B R, ^Jl"!NVINOSHimS^S3iavyan"'LIBRARIES^SMITHS0NIAN~'lNSTITUTI0N^NOIin L '~^' ^ [I ^ d 2 OJ .^ . ° /<SS^ CD /<dSi^ 2 .^^^. ro /l^2l^!^ 2 /<^ > ^'^^ ^ ..... ^ - m x^^osvAVix ^' m S SMITHSONIAN INSTITUTION — NOIlfliliSNrNVINOSHimS^SS iyvyan~LIBR/ S "^ ^ ^ c/> z 2 O _ Xto Iz JI_NVIN0SH1I1/MS^S3 I a Vd a n^LI B RAR I ES'^SMITHSONIAN JNSTITUTION "^NOlin Z -I 2 _j 2 _j S SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVaan LI BR/ 2: r- — 2 r- z NVINOSHiltNS ^1 S3 I MVy I 8 n~L B R AR I Es'^SMITHSONIAN'iNSTITUTIOn'^ NOlin ^^^>^ CO z w • z i ^^ > ^ s smithsonian_institution NoiiniiiSNi to NviNosHiiws'^ss I dVH a n^Li br; <n / .* -5^ \^A DO « ^\t PUBLISHED BI-MONTHLY ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. -

Marie Sjögren Thesis
Energy metabolism as a target for new treatment strategies in Huntington’s disease Sjögren, Marie 2019 Document Version: Publisher's PDF, also known as Version of record Link to publication Citation for published version (APA): Sjögren, M. (2019). Energy metabolism as a target for new treatment strategies in Huntington’s disease. Lund University: Faculty of Medicine. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 10. Oct. 2021 MARIE SJÖGREN Energy metabolism as a target new for treatment strategies disease in Huntington’s Energy metabolism as -

The Archaeological Context of Sea Level Change in Prehistoric South Wales
Changing Tides: The Archaeological Context of Sea Level Change in Prehistoric South Wales Volume 1 Thesis submitted for the degree of PhD Cardiff University Department of Archaeology and Conservation School of History Archaeology and Religion September 2018 Rhiannon Philp DECLARATION This work has not been submitted in substance for any other degree or award at this or any other university or place of learning, nor is being submitted concurrently in candidature for any degree or other award. Signed (candidate) Date: 28/09/18 STATEMENT 1 This thesis is being submitted in partial fulfilment of the requirements for the degree of PhD. Signed (candidate) Date: 28/09/18 STATEMENT 2 This thesis is the result of my own independent work/investigation, except where otherwise stated, and the thesis has not been edited by a third party beyond what is permitted by Cardiff University’s Policy on the Use of Third Party Editors by Research Degree Students. Other sources are acknowledged by explicit references. The views expressed are my own. Signed (candidate) Date: 28/09/18 STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available online in the University’s Open Access repository and for inter-library loan, and for the title and summary to be made available to outside organisations. Signed (candidate) Date: 28/09/18 Summary Evidence for the effects of past sea level change can be found in the submerged landscapes within the intertidal zone of Britain. Such evidence is particularly prevalent in Wales, but understanding the rate at which these changes occurred during prehistory is limited, due to a dearth of palaeoenvironmental, archaeological and temporal data. -

C:\Documents and Settings\Owner\Desktop
A is for Asimov Annotated index of the 22 Magazine of Fantasy and Science Fiction anthology collections. Prepared by Caroline Minuscule for The Isaac Asimov Project The Thunder Child SF & Fantasy Webzine To be used for research, reference and review First edition: October 29, 2008 The twenty two books in the F & SF series indexed here: Title Code Version 1. Fact and Fancy (1962) FF PB 2. View from a Height (1963) VH HB 3. Adding a Dimension (1964) AD PB 4. Of Time, Space & Other Things (1965) TS PB 5. From Earth to Heaven (1966) EH PB 6. Science, Numbers and I (1968) SN PB 7. The Solar System and Back (1970) SS PB 8. The Stars in Their Courses (1971) SC PB 9. Left Hand of the Electron (1972) LE PB 10. The Tragedy of the Moon (1973) TM PB 11. Of Matters Great & Small (1975) GS PB 12. The Planet That Wasn't (1976) PW HB 13. Quasar, Quasar, Burning Bright (1977) QQ HB 14. The Road to Infinity (1979) RI PB 15. The Sun Shines Bright (1981) SB HB 16. Counting the Eons (1983) CE HB 17. X Stands for Unknown (1984) XU HB 18. The Subatomic Monster (1985) SM PB 19. Far as Human Eye Could See (1987) HE HB 20. The Relativity of Wrong (1988) RW HB 21. Out of The Everywhere (1990) OE PB 22. The Secret of The Universe (1990) SU HB Repackaging: Asimov on Astronomy Asimov on Chemistry Asimov on Physics Asimov on Numbers Asimov on Science These are compiled from essays previously published in the above books. -
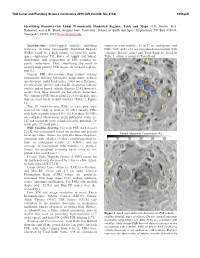
Identifying Resource-Rich Lunar Permanently Shadowed Regions: Table and Maps
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 1054.pdf Identifying Resource-rich Lunar Permanently Shadowed Regions: Table and Maps. H.M. Brown, M.S. Robinson, and A.K. Boyd, Arizona State University, School of Earth and Space Exploration, PO Box 873603, Tempe AZ, 85287-3603 [email protected] Introduction: Cold-trapped volatiles, including consistent with volatiles, 13 to 17 are ambiguous, and water-ice, in lunar Permanently Shadowed Regions PSRs with ranks <12 are considered inconsistent with (PSRs) could be a high priority resource for future volatiles. Dataset scores and Total Rank are listed in space exploration [1]. Rates of supply and burial, Table 1, which is sorted by Total Rank. distribution, and composition of PSR volatiles are poorly understood. Thus, amplifying the need to identify high priority PSR targets for focused explora- tion [1]. Current PSR observations from remote sensing instruments utilizing bolometric temperature, neutron spectroscopy, radar backscatter, 1064 nm reflectance, far-ultraviolet spectra, and near-IR absorption indicate surface and/or buried volatile deposits [2-8]; however, results from these datasets are not always correlated. We compared PSR observations [2-14] to identify sites that are most likely to host volatiles (Table 1, Figure 1). The 10 largest-by-area PSRs at each pole were selected for study as well as 35 other smaller PSRs with high scientific interest [10-13]. For these 55 PSRs we compiled observations from published works [2- 14] and scored them by volatile/resource potential (28 south pole, 27 north pole). PSR Volatiles Scoring: For each PSR, each dataset [2-14] was categorized based on median and percent Annual Maximum Temperature (K) coverage values. -
(1846–1920). Russian Jeweller, of French Descent. He Achieved Fame
Fabricius ab Aquapendente, Hieronymus (Geronimo Fabrizi) (1533–1619). Italian physician, born at Aquapendente, near Orvieto. He studied medicine under *Fallopio at Padua and succeeded F him as professor of surgery and anatomy 1562– 1613. He became actively involved in building Fabergé, Peter Carl (1846–1920). Russian jeweller, the university’s magnificent anatomical theatre, of French descent. He achieved fame by the ingenuity which is preserved today. He acquired fame as a and extravagance of the jewelled objects (especially practising physician and surgeon, and made extensive Easter eggs) he devised for the Russian nobility and contributions to many fields of physiology and the tsar in an age of ostentatious extravagance which medicine, through his energetic skills in dissection ended on the outbreak of World War I. He died in and experimentation. He wrote works on surgery, Switzerland. discussing treatments for different sorts of wounds, and a major series of embryological studies, illustrated Fabius, Laurent (1946– ). French socialist politician. by detailed engravings. His work on the formation of He was Deputy 1978–81, 1986– , Minister for the foetus was especially important for its discussion Industry and Research 1983–84, Premier of France of the provisions made by nature for the necessities 1984–86, Minister of Economics 2000–02 and of the foetus during its intra-uterine life. The medical Foreign Minister 2012–16, and President of the theory he offered to explain the development of eggs Constitutional Council 2016– . and foetuses, however, was in the tradition of *Galen. Fabricius is best remembered for his detailed studies Fabius Maximus Verrocosus Cunctator, Quintus of the valves of the veins. -

Testing Lunar Permanently Shadowed Regions for Water Ice: LEND Results from LRO A
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117, E00H26, doi:10.1029/2011JE003971, 2012 Testing lunar permanently shadowed regions for water ice: LEND results from LRO A. B. Sanin,1 I. G. Mitrofanov,1 M. L. Litvak,1 A. Malakhov,1 W. V. Boynton,2 G. Chin,3 G. Droege,2 L. G. Evans,4 J. Garvin,3 D. V. Golovin,1 K. Harshman,2 T. P. McClanahan,3 M. I. Mokrousov,1 E. Mazarico,3 G. Milikh,5 G. Neumann,3 R. Sagdeev,5 D. E. Smith,6 R. D. Starr,7 and M. T. Zuber6 Received 22 September 2011; revised 19 April 2012; accepted 30 April 2012; published 15 June 2012. [1] We use measurements from the Lunar Exploration Neutron Detector (LEND) collimated sensors during more than one year of the mapping phase of NASA’s Lunar Reconnaissance Orbiter (LRO) mission to make estimates of the epithermal neutron flux within known large Permanently Shadowed Regions (PSRs). These are compared with the local neutron background measured outside PSRs in sunlit regions. Individual and collective analyses of PSR properties have been performed. Only three large PSRs, Shoemaker and Cabeus in the south and Rozhdestvensky U in the north, have been found to manifest significant neutron suppression. All other PSRs have much smaller suppression, only a few percent, if at all. Some even display an excess of neutron emission in comparison to the sunlit vicinity around them. Testing PSRs collectively, we have not found any average suppression for them. Only the group of 18 large PSRs, with area >200 km2, show a marginal effect of small average suppression, 2%, with low statistical confidence. -

Lunar 1000 Challenge List
LUNAR 1000 CHALLENGE A B C D E F G H I LUNAR PROGRAM BOOKLET LOG 1 LUNAR OBJECT LAT LONG OBJECTIVE RUKL DATE VIEWED BOOK PAGE NOTES 2 Abbot 5.6 54.8 37 3 Abel -34.6 85.8 69, IV Libration object 4 Abenezra -21.0 11.9 55 56 5 Abetti 19.9 27.7 24 6 Abulfeda -13.8 13.9 54 45 7 Acosta -5.6 60.1 49 8 Adams -31.9 68.2 69 9 Aepinus 88.0 -109.7 Libration object 10 Agatharchides -19.8 -30.9 113 52 11 Agrippa 4.1 10.5 61 34 12 Airy -18.1 5.7 63 55, 56 13 Al-Bakri 14.3 20.2 35 14 Albategnius -11.2 4.1 66 44, 45 15 Al-Biruni 17.9 92.5 III Libration object 16 Aldrin 1.4 22.1 44 35 17 Alexander 40.3 13.5 13 18 Alfraganus -5.4 19.0 46 19 Alhazen 15.9 71.8 27 20 Aliacensis -30.6 5.2 67 55, 65 21 Almanon -16.8 15.2 55 56 22 Al-Marrakushi -10.4 55.8 48 23 Alpetragius -16.0 -4.5 74 55 24 Alphonsus -13.4 -2.8 75 44, 55 25 Ameghino 3.3 57.0 38 26 Ammonius -8.5 -0.8 75 44 27 Amontons -5.3 46.8 48 28 Amundsen -84.5 82.8 73, 74, V Libration object 29 Anaxagoras 73.4 -10.1 76 4 30 Anaximander 66.9 -51.3 2 31 Anaximenes 72.5 -44.5 3 32 Andel -10.4 12.4 45 33 Andersson -49.7 -95.3 VI Libration object 34 Angstrom 29.9 -41.6 19 35 Ansgarius -12.7 79.7 49, IV Libration object 36 Anuchin -49.0 101.3 V Libration object 37 Anville 1.9 49.5 37 38 Apianus -26.9 7.9 55 56 39 Apollonius 4.5 61.1 2 38 40 Arago 6.2 21.4 44 35 41 Aratus 23.6 4.5 22 42 Archimedes 29.7 -4.0 78 22, 12 43 Archytas 58.7 5.0 76 4 44 Argelander -16.5 5.8 63 56 45 Ariadaeus 4.6 17.3 35 46 Aristarchus 23.7 -47.4 122 18 47 Aristillus 33.9 1.2 69 12 48 Aristoteles 50.2 17.4 48 5 49 Armstrong 1.4 25.0 44 -

Natura Revista Ştiinţifica De Popularizare
NATURA REVISTA ŞTIINŢIFICA DE POPULARIZARE PUBLICATĂ PRIN ÎNGRIJIREA D-LOR G. ŢIŢEICA şi G. G. LONGINESCU DOCTOR ÎN ŞTIINŢE, PROFESOR DOCTOR ÎN ŞTIINŢE, PROFESOR UNIVERSITAR UNIVERSITAR ANUL IX 1913-1914 BUCUREŞTI TIPOGRAFIA „GUTENBERG“, J. GOBL S-sori 20. - STRADA DOAMNEI. — 20 (Biserica Kalinderu) ABONAMENTUL pe un an (10 numere) 15 Octomvrie—15 Iulie, 5 Lei. APARE LA 15 A FIECĂREI LUNI SUI! ÎNGRIJIHKA D-I.Ofi G. ŢIŢEICA, doctor în ştiinţe, profesor universitar. G. G. LONGINESCU, doctor în ştiinţe, profesor universitar. Pentru orice priveşte revista a se adresa d-iui O, Ţiţeica. str. Scaune, 33. Anul III, IV, V, VI, VII, VIII şi IX in volume broşate costă 6 lei fiecare. Anul I costă 8 lei. iar anul II costă 7 lei, şi se găsesc la adresa de mai sus. 1 NATURA REVISTĂ ŞTIINŢIFICĂ DE POPULARIZARE PUBLICATĂ SUB ÎNGRIJIREA D-LOR G. ŢIŢEICA şi G. G. LONGINESCU VOLUM UL IX OCTOMVRIE 1913 - IULIE 1914 BUCUREŞTI TIPOGRAFIA „GUTENBERG“ JOSEPH GÔBL S-so r i 20. — STRADA DOAMNEI. — 20. 1913 CIRCULAŢIA REPEDE IN ORAŞELE MARI CARACTERUL ORAŞELOR MARI. - MIJLOACE DE A ÎNLESNI CIRCULAŢIA. - CIRCULAŢIA AERIANĂ ŞI SUBTERANĂ. — CONSTRUCŢIA LINIILOR SUBTERANE. — FINAN ŢELE ŞI ADMINISTRAŢIA METROPOLITANELOR. — PROECTE PENTRU VIITOR I. Un jurnalist scria pe la 1889: »Hm stat douăzeci de mi- »nute pe trotoar în Oxford Street1), ca şi ţăranul lui Hora- «ţiu care, ca să treacă un fluviu, aşteptă să se scurgă toată »apa. Dar vai! fluviul de trăsuri curgea mereu! In fine, un »grup de lucrători, care treceâ pe lângă mine, crezu că a »găsit o trecere şi se aruncă în şivoiu. -

'U Gws THUNDER SALUTE AS NEW COMMANDANT COMES to NAVAL STATION
rroa 8. F.I Honolulan, Mch. 26. For S. F.t - Manchuria, Mch. 25 From Yaneeavers -- ; Zealandla, Mar. 26 v Fr Yaneotrert ; Marama, Mar. 25 ; Evening Bulletin. 1882, No. 5302. Est ::; 14 -H- 1013 Hawaiian Star. Vol. XX, No. C54S. PAGES. ONOLULU, TERRITORY OF HAWAII, MONDAY, MAKCII 24, 14 PAGES. price five cnrca -- -J- i r r T- n J. IMP LJ wit-- :. J .O v I ! 'U gws THUNDER SALUTE AS NEW COMMANDANT COMES TO NAVAL STATION I i . U La w-- 3 O , ' "' :".x .''-- ',' i - ' -- V; ?,'. - ' , W-- 4- Thrc,-,:- Terrific Storm Sweeps !i Fifty-thousa- nd Dollar Loss in Four States, Ccu;:r,a ; ' - Hct Claze Yesterday : - Immense Lees . v. : t Afternoon ; . 240 v Tn : ruuirio"f: i'-'Tf""- ".. AND k .TtEHOUSE J linnnUU ;,. i - -- " ARE DESTROYED V V. rr , Nebraskr,v:v. rr.. L - Direction of Wind; Saves Vt JIlinDisVi: I:: I:;.:! . ru; i ;Ian3 rr ? ' of Un Cvwuwti j : ti Has J riarrc;Et::pir'!ij! (As3oc5cd rr:-- 3 CzV.z) OMAHA, fhb.f T.v? (Fpecial fitar-Eulkt-ln Tirch Wireless) V: h for rrany , IIILO, Hawafl,-Marc- 24 A" Tierce. worst, tornaij yt:ra ii .': Admirals Cowles ('o. 1) and Moore (Xo. 2) and the officers of the naTal station '"and marine; barracks, pho- c . ng rncst :.. aft--. ' ' '" ' state and one cf the fire at OJaa yesterday - - ' " tographed this morning at naTal station ceremonies. ' ' . ' ". ..' ercoon, the second, of two disastrous to life in the history cf t: :: ' ' over the isction cf c:-'- .r conflagrations on this' Island : within broke t: a few weeks, wiped outs the Olaa pi r no ffri 7 near Council ClufTs, Ir.va.