Supplementary Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of California, San Diego
UNIVERSITY OF CALIFORNIA, SAN DIEGO The post-terminal differentiation fate of RNAs revealed by next-generation sequencing A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Biomedical Sciences by Gloria Kuo Lefkowitz Committee in Charge: Professor Benjamin D. Yu, Chair Professor Richard Gallo Professor Bruce A. Hamilton Professor Miles F. Wilkinson Professor Eugene Yeo 2012 Copyright Gloria Kuo Lefkowitz, 2012 All rights reserved. The Dissertation of Gloria Kuo Lefkowitz is approved, and it is acceptable in quality and form for publication on microfilm and electronically: __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ Chair University of California, San Diego 2012 iii DEDICATION Ma and Ba, for your early indulgence and support. Matt and James, for choosing more practical callings. Roy, my love, for patiently sharing the ups and downs of this journey. iv EPIGRAPH It is foolish to tear one's hair in grief, as though sorrow would be made less by baldness. ~Cicero v TABLE OF CONTENTS Signature Page .............................................................................................................. iii Dedication .................................................................................................................... -

Supplementary Materials: Evaluation of Cytotoxicity and Α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
Supplementary Materials: Evaluation of cytotoxicity and α-glucosidase inhibitory activity of amide and polyamino-derivatives of lupane triterpenoids Oxana B. Kazakova1*, Gul'nara V. Giniyatullina1, Akhat G. Mustafin1, Denis A. Babkov2, Elena V. Sokolova2, Alexander A. Spasov2* 1Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences, 71, pr. Oktyabrya, 450054 Ufa, Russian Federation 2Scientific Center for Innovative Drugs, Volgograd State Medical University, Novorossiyskaya st. 39, Volgograd 400087, Russian Federation Correspondence Prof. Dr. Oxana B. Kazakova Ufa Institute of Chemistry of the Ufa Federal Research Centre of the Russian Academy of Sciences 71 Prospeсt Oktyabrya Ufa, 450054 Russian Federation E-mail: [email protected] Prof. Dr. Alexander A. Spasov Scientific Center for Innovative Drugs of the Volgograd State Medical University 39 Novorossiyskaya st. Volgograd, 400087 Russian Federation E-mail: [email protected] Figure S1. 1H and 13C of compound 2. H NH N H O H O H 2 2 Figure S2. 1H and 13C of compound 4. NH2 O H O H CH3 O O H H3C O H 4 3 Figure S3. Anticancer screening data of compound 2 at single dose assay 4 Figure S4. Anticancer screening data of compound 7 at single dose assay 5 Figure S5. Anticancer screening data of compound 8 at single dose assay 6 Figure S6. Anticancer screening data of compound 9 at single dose assay 7 Figure S7. Anticancer screening data of compound 12 at single dose assay 8 Figure S8. Anticancer screening data of compound 13 at single dose assay 9 Figure S9. Anticancer screening data of compound 14 at single dose assay 10 Figure S10. -

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research, -

Effects of Chromosome Specific Introgression in Upland Cotton on Fiber and Agronomic Traits
Genetics: Published Articles Ahead of Print, published on December 30, 2005 as 10.1534/genetics.105.053371 1 Effects of Chromosome Specific Introgression in Upland Cotton on Fiber and Agronomic Traits * 1 * † * Sukumar Saha, Johnie N. Jenkins, Jixiang Wu, Jack C. McCarty, * ‡ § †† Osman A. Gutiérrez, Richard G. Percy, Roy G. Cantrell, and David M. Stelly *United States Department of Agriculture-Agriculture Research Service, Crop Science Research † Laboratory, Mississippi State, Mississippi 39762, Department of Plant and Soil Sciences, ‡ Mississippi State University, Mississippi State, Mississippi 39762, United States Department of Agriculture-Agriculture Research Service, Maricopa Agricultural Center, Maricopa, Arizona § †† 85239, Cotton Incorporated, Raleigh, North Carolina 27513, and Department of Soil and Crop Sciences, Texas A&M University, College Station, Texas 77843 Disclaimer: Mention of trademark or proprietary product does not constitute a guarantee or warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable. 2 Running head: Evaluation of fiber and agronomic traits using chromosome substitution lines Key words: Chromosome substitution, Cotton, QTL, Germplasm, Mapping 1 Corresponding author: United States Department of Agriculture-Agriculture Research Service, Crop Science Research Laboratory, 810 Highway 12 East, Mississippi State, Mississippi 39762- 5367. E-mail: [email protected] 3 ABSTRACT Interspecific chromosome substitution is among the most powerful means of introgression and steps toward quantitative trait locus (QTL) identification. By reducing the genetic “noise” from other chromosomes, it greatly empowers the detection of genetic effects by specific chromosomes on quantitative traits. Here, we report on such results for 14 cotton lines (CS-B) with specific chromosomes or chromosome arms from G. -

Structural Characterization of the Human Eukaryotic Initiation Factor 3 Protein Complex by Mass Spectrometry*□S
Supplemental Material can be found at: http://www.mcponline.org/cgi/content/full/M600399-MCP200 /DC1 Research Structural Characterization of the Human Eukaryotic Initiation Factor 3 Protein Complex by Mass Spectrometry*□S Eugen Damoc‡, Christopher S. Fraser§, Min Zhou¶, Hortense Videler¶, Greg L. Mayeurʈ, John W. B. Hersheyʈ, Jennifer A. Doudna§, Carol V. Robinson¶**, and Julie A. Leary‡ ‡‡ Protein synthesis in mammalian cells requires initiation The initiation phase of eukaryotic protein synthesis involves factor eIF3, an ϳ800-kDa protein complex that plays a formation of an 80 S ribosomal complex containing the initi- Downloaded from central role in binding of initiator methionyl-tRNA and ator methionyl-tRNAi bound to the initiation codon in the mRNA to the 40 S ribosomal subunit to form the 48 S mRNA. This is a multistep process promoted by proteins initiation complex. The eIF3 complex also prevents pre- called eukaryotic initiation factors (eIFs).1 Currently at least 12 mature association of the 40 and 60 S ribosomal subunits eIFs, composed of at least 29 distinct subunits, have been and interacts with other initiation factors involved in start identified (1). Mammalian eIF3, the largest initiation factor, is a codon selection. The molecular mechanisms by which multisubunit complex with an apparent molecular mass of www.mcponline.org eIF3 exerts these functions are poorly understood. Since ϳ800 kDa. This protein complex plays an essential role in its initial characterization in the 1970s, the exact size, translation by binding directly to the 40 S ribosomal subunit composition, and post-translational modifications of and promoting formation of the 43 S preinitiation complex ⅐ ⅐ mammalian eIF3 have not been rigorously determined. -
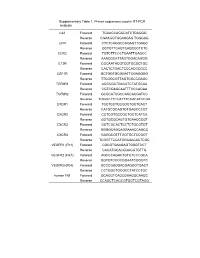
Supplementary Table 1. Primer Sequences Used in RT-PCR Analysis
Supplementary Table 1. Primer sequences used in RT-PCR analysis L32 Forward TGAAGCAGGCATCTGAGGG Reverse CGAAGGTGGAAGAG TGGGAG LIFR Forward CTCTCAGGCCAGAGTTGAGC Reverse GCTGTTCAGTCAGCCCTCTC CCR2 Forward TGTCTTCCCTGAATTGAGCC Reverse AAACGCATTAGTGGACAGGG IL12R Forward CGCAATACGTCGTGCGCTGC Reverse CACTCTGACTCCCACGCGCC CSF1R Forward GCTGGTGCGGATTCGAGGGG Reverse TTCGGCGTTAGTGGCCGAGC TGFbR1 Forward ACGCGCTGACATCTATGCAA Reverse CGTCGAGCAATTTCCCAGAA TGFbR2 Forward GCGCATCGCCAGCACGATCC Reverse TGGGCTTCCATTTCCACATCCGA CXCR1 Forward TCCTCCTGCCGCTGCTCACT Reverse CATGCGCAGTGTGAGCCCGT CXCR2 Forward CCTCGTGCCGCTGCTCATCA Reverse GGTGCGCAGTGTGAACCCGT CXCR3 Forward GGTCGCACTGCTCTGCGTGT Reverse GGGGCAGCAGGAAACCAGCC CXCR4 Forward GAGGCGTTTGGTGCTCCGGT Reverse TCGGTTCCATGGCAACACTCGC VEGFR1 (Flt1) Forward CGCGTGAAGAGTGGGTCCT Reverse CACATGCACGGAGGTGTTG VEGFR2 (Flk1) Forward AGCCCAGACTGTGTCCCGCA Reverse GGTGTCCGCGGAATCGGGTC VEGFR3 (Flt4) Forward GCCCGAGGACGAGGGTGACT Reverse CCTGGCTGCGCCTATCCTGC human Flt1 Forward GCACGTCAGCGAAGGCAAGC Reverse CCAGCTCAGCGTGGTCGTAGG Supplementary Table 2. List of putative miR-200 target genes amplified in human cancers amplified in cancers (Tumorscape)a Gene Symbol Title various cancers NSCLC PCT Errfi1 ERBB receptor feedback inhibitor 1 - yes 0.94 D11Bwg0517e DNA segment, Chr 11, Brigham & Women's Genetics 0517 expressed - yes 0.91 Pvrl4 poliovirus receptor-related 4 yes - 0.91 Mecp2 methyl CpG binding protein 2 yes yes 0.9 Jun Jun oncogene yes - 0.88 Prdm16 PR domain containing 16 - yes 0.88 Flt1 FMS-like tyrosine kinase 1 yes -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Dramatic Nucleolar Dispersion in the Salivary Gland of Schwenkfeldina Sp
www.nature.com/scientificreports OPEN Dramatic nucleolar dispersion in the salivary gland of Schwenkfeldina sp. (Diptera: Sciaridae) José Mariano Amabis & Eduardo Gorab * Micronucleoli are among the structures composing the peculiar scenario of the nucleolus in salivary gland nuclei of dipterans representative of Sciaridae. Micronucleolar bodies contain ribosomal DNA and RNA, are transcriptionally active and may appear free in the nucleoplasm or associated with specifc chromosome regions in salivary gland nuclei. This report deals with an extreme case of nucleolar fragmentation/dispersion detected in the salivary gland of Schwenkfeldina sp. Such a phenomenon in this species was found to be restricted to cell types undergoing polyteny and seems to be diferentially controlled according to the cell type. Furthermore, transcriptional activity was detected in virtually all the micronucleolar bodies generated in the salivary gland. The relative proportion of the rDNA in polytene and diploid tissues showed that rDNA under-replication did not occur in polytene nuclei suggesting that the nucleolar and concomitant rDNA dispersion in Schwenkfeldina sp. may refect a previously hypothesised process in order to counterbalance the rDNA loss due to the under-replication. The chromosomal distribution of epigenetic markers for the heterochromatin agreed with early cytological observations in this species suggesting that heterochromatin is spread throughout the chromosome length of Schwenkfeldina sp. A comparison made with results from another sciarid species argues for a role played by the heterochromatin in the establishment of the rDNA topology in polytene nuclei of Sciaridae. Transcriptional activity of ribosomal RNA (rRNA) genes in specifc chromosome regions is the primary event for the local assembly of the nucleolus, the starting site of ribosome biogenesis. -

RNA-Binding Proteins in Human Oogenesis: Balancing Differentiation and Self-Renewal in the Female Fetal Germline
Stem Cell Research 21 (2017) 193–201 Contents lists available at ScienceDirect Stem Cell Research journal homepage: www.elsevier.com/locate/scr RNA-binding proteins in human oogenesis: Balancing differentiation and self-renewal in the female fetal germline Roseanne Rosario a, Andrew J. Childs b, Richard A. Anderson a,⁎ a MRC Centre for Reproductive Health, Queen's Medical Research Institute, University of Edinburgh, 47 Little France Crescent, Edinburgh EH16 4TJ, UK b Department of Comparative Biomedical Sciences, The Royal Veterinary College, London NW1 0TU, UK article info abstract Article history: Primordial germ cells undergo three significant processes on their path to becoming primary oocytes: the initia- Received 7 October 2016 tion of meiosis, the formation and breakdown of germ cell nests, and the assembly of single oocytes into primor- Received in revised form 29 March 2017 dial follicles. However at the onset of meiosis, the germ cell becomes transcriptionally silenced. Consequently Accepted 13 April 2017 translational control of pre-stored mRNAs plays a central role in coordinating gene expression throughout the re- Available online 18 April 2017 mainder of oogenesis; RNA binding proteins are key to this regulation. In this review we examine the role of ex- Keywords: emplars of such proteins, namely LIN28, DAZL, BOLL and FMRP, and highlight how their roles during germ cell Germ cell differentiation development are critical to oogenesis and the establishment of the primordial follicle pool. RNA binding proteins © 2017 The Authors. -

4-6 Weeks Old Female C57BL/6 Mice Obtained from Jackson Labs Were Used for Cell Isolation
Methods Mice: 4-6 weeks old female C57BL/6 mice obtained from Jackson labs were used for cell isolation. Female Foxp3-IRES-GFP reporter mice (1), backcrossed to B6/C57 background for 10 generations, were used for the isolation of naïve CD4 and naïve CD8 cells for the RNAseq experiments. The mice were housed in pathogen-free animal facility in the La Jolla Institute for Allergy and Immunology and were used according to protocols approved by the Institutional Animal Care and use Committee. Preparation of cells: Subsets of thymocytes were isolated by cell sorting as previously described (2), after cell surface staining using CD4 (GK1.5), CD8 (53-6.7), CD3ε (145- 2C11), CD24 (M1/69) (all from Biolegend). DP cells: CD4+CD8 int/hi; CD4 SP cells: CD4CD3 hi, CD24 int/lo; CD8 SP cells: CD8 int/hi CD4 CD3 hi, CD24 int/lo (Fig S2). Peripheral subsets were isolated after pooling spleen and lymph nodes. T cells were enriched by negative isolation using Dynabeads (Dynabeads untouched mouse T cells, 11413D, Invitrogen). After surface staining for CD4 (GK1.5), CD8 (53-6.7), CD62L (MEL-14), CD25 (PC61) and CD44 (IM7), naïve CD4+CD62L hiCD25-CD44lo and naïve CD8+CD62L hiCD25-CD44lo were obtained by sorting (BD FACS Aria). Additionally, for the RNAseq experiments, CD4 and CD8 naïve cells were isolated by sorting T cells from the Foxp3- IRES-GFP mice: CD4+CD62LhiCD25–CD44lo GFP(FOXP3)– and CD8+CD62LhiCD25– CD44lo GFP(FOXP3)– (antibodies were from Biolegend). In some cases, naïve CD4 cells were cultured in vitro under Th1 or Th2 polarizing conditions (3, 4). -

Manuscript for Proximity Interactome Analysis of Lassa
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.16.452739; this version posted July 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Main Manuscript for 2 Proximity interactome analysis of Lassa polymerase reveals 3 eRF3a/GSPT1 as a druggable target for host directed antivirals. 4 Jingru Fang1,2, Colette Pietzsch3,4, George Tsaprailis5, Gogce Crynen5, Kelvin Frank Cho6, Alice 5 Y. Ting7,8, Alexander Bukreyev3,4,9, Erica Ollmann Saphire2* and Juan Carlos de la Torre1* 6 1 Department of Immunology and Microbiology, Scripps Research, La Jolla CA 92037 7 2La Jolla Institute for Immunology, La Jolla CA 92037 8 3Department of Pathology, University of Texas Medical Branch, Galveston, TX 77550 9 4Galveston National Laboratory, University of Texas Medical Branch, Galveston, TX 77550 10 5Proteomics Core, Scripps Research, Jupiter, FL 33458 11 6Cancer Biology Program, Stanford University, Stanford, CA 94305 12 7Department of Genetics, Department of Biology and Department of Chemistry, Stanford 13 University, Stanford, CA 94305 14 8Chan Zuckerberg Biohub, San Francisco, CA 94158 15 9Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, 16 TX 77550 17 *Erica Ollmann Saphire; Juan Carlos de la Torre. 18 Email: [email protected] and [email protected] 19 Author Contributions: J.F., E.O.S., and J.C.T designed research; J.F. performed all non-BSL4 20 experiments and analyzed data; C.P. -

Crystal Structure of the Eukaryotic 60S Ribosomal Subunit in Complex with Initiation Factor 6
Research Collection Doctoral Thesis Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6 Author(s): Voigts-Hoffmann, Felix Publication Date: 2012 Permanent Link: https://doi.org/10.3929/ethz-a-007303759 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library ETH Zurich Dissertation No. 20189 Crystal Structure of the Eukaryotic 60S Ribosomal Subunit in Complex with Initiation Factor 6 A dissertation submitted to ETH ZÜRICH for the degree of Doctor of Sciences (Dr. sc. ETH Zurich) presented by Felix Voigts-Hoffmann MSc Molecular Biotechnology, Universität Heidelberg born April 11, 1981 citizen of Göttingen, Germany accepted on recommendation of Prof. Dr. Nenad Ban (Examiner) Prof. Dr. Raimund Dutzler (Co-examiner) Prof. Dr. Rudolf Glockshuber (Co-examiner) 2012 blank page ii Summary Ribosomes are large complexes of several ribosomal RNAs and dozens of proteins, which catalyze the synthesis of proteins according to the sequence encoded in messenger RNA. Over the last decade, prokaryotic ribosome structures have provided the basis for a mechanistic understanding of protein synthesis. While the core functional centers are conserved in all kingdoms, eukaryotic ribosomes are much larger than archaeal or bacterial ribosomes. Eukaryotic ribosomal rRNA and proteins contain extensions or insertions to the prokaryotic core, and many eukaryotic proteins do not have prokaryotic counterparts. Furthermore, translation regulation and ribosome biogenesis is much more complex in eukaryotes, and defects in components of the translation machinery are associated with human diseases and cancer.