Phylogeny of the Anomura (Decapoda, Crustacea): Spermatozoa And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project
High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project AEA Technology, Environment Contract: W/35/00632/00/00 For: The Department of Trade and Industry New & Renewable Energy Programme Report issued 30 August 2002 (Version with minor corrections 16 September 2002) Keith Hiscock, Harvey Tyler-Walters and Hugh Jones Reference: Hiscock, K., Tyler-Walters, H. & Jones, H. 2002. High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Report from the Marine Biological Association to The Department of Trade and Industry New & Renewable Energy Programme. (AEA Technology, Environment Contract: W/35/00632/00/00.) Correspondence: Dr. K. Hiscock, The Laboratory, Citadel Hill, Plymouth, PL1 2PB. [email protected] High level environmental screening study for offshore wind farm developments – marine habitats and species ii High level environmental screening study for offshore wind farm developments – marine habitats and species Title: High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Contract Report: W/35/00632/00/00. Client: Department of Trade and Industry (New & Renewable Energy Programme) Contract management: AEA Technology, Environment. Date of contract issue: 22/07/2002 Level of report issue: Final Confidentiality: Distribution at discretion of DTI before Consultation report published then no restriction. Distribution: Two copies and electronic file to DTI (Mr S. Payne, Offshore Renewables Planning). One copy to MBA library. Prepared by: Dr. K. Hiscock, Dr. H. Tyler-Walters & Hugh Jones Authorization: Project Director: Dr. Keith Hiscock Date: Signature: MBA Director: Prof. S. Hawkins Date: Signature: This report can be referred to as follows: Hiscock, K., Tyler-Walters, H. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
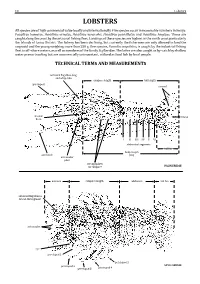
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Systematics, Phylogeny, and Taphonomy of Ghost Shrimps (Decapoda): a Perspective from the Fossil Record
73 (3): 401 – 437 23.12.2015 © Senckenberg Gesellschaft für Naturforschung, 2015. Systematics, phylogeny, and taphonomy of ghost shrimps (Decapoda): a perspective from the fossil record Matúš Hyžný *, 1, 2 & Adiël A. Klompmaker 3 1 Geological-Paleontological Department, Natural History Museum Vienna, Burgring 7, 1010 Vienna, Austria; Matúš Hyžný [hyzny.matus@ gmail.com] — 2 Department of Geology and Paleontology, Faculty of Natural Sciences, Comenius University, Mlynská dolina, Ilkovičova 6, SVK-842 15 Bratislava, Slovakia — 3 Florida Museum of Natural History, University of Florida, 1659 Museum Road, PO Box 117800, Gaines- ville, FL 32611, USA; Adiël A. Klompmaker [[email protected]] — * Correspond ing author Accepted 06.viii.2015. Published online at www.senckenberg.de/arthropod-systematics on 14.xii.2015. Editor in charge: Stefan Richter. Abstract Ghost shrimps of Callianassidae and Ctenochelidae are soft-bodied, usually heterochelous decapods representing major bioturbators of muddy and sandy (sub)marine substrates. Ghost shrimps have a robust fossil record spanning from the Early Cretaceous (~ 133 Ma) to the Holocene and their remains are present in most assemblages of Cenozoic decapod crustaceans. Their taxonomic interpretation is in flux, mainly because the generic assignment is hindered by their insufficient preservation and disagreement in the biological classification. Fur- thermore, numerous taxa are incorrectly classified within the catch-all taxonCallianassa . To show the historical patterns in describing fos- sil ghost shrimps and to evaluate taphonomic aspects influencing the attribution of ghost shrimp remains to higher level taxa, a database of all fossil species treated at some time as belonging to the group has been compiled: 250 / 274 species are considered valid ghost shrimp taxa herein. -

Proceedings of the United States National Museum
descriptions of a new genus and forty-six new spp:cies of crusta(jeans of the family GALA- theid.e, with a list of the known marine SPECIES. By James E. Benedict, Assistant Curator of Marine Invertebrates. The collection of Galatheids in the United States National Museum, upon which this paper is based, began with the first dredgings of the U. S. Fish Commission steamer Albatross in 1883, and has grown as that busy ship has had opportunit}' to dredge. During the first period of its work many of the species taken were identical with those found by the U. S. Coast Survey steamer JBlake, afterwards described by A. Milne-Edwards, and in addition several new species were collected. During the voyage of the Albatross to the Pacific Ocean through the Straits of Magellan interesting addi- tions were made to the collection. Since then the greater part of the time spent by the Albatross at sea has been in Alaskan waters, where Galatheids do not seem to abound. However, occasional cruises else- where have greatly enriched the collection, notably three—one in the Gulf of California, one to the Galapagos Islands, and one to the coast of Japan and southward. The U. S. National Museum has received a number of specimens from the Museum of Natural History, Paris, and also from the Indian Museum, Calcutta. The literature of the deep-sea Galatheidie from the nature of the case is not greatly scattered. The first considerate number of species were described by A. Milne-Edwards from dredgings made by the Blake in the West Indian region. -

Deep-Water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada
Bull. Natl. Mus. Nat. Sci., Ser. A, 46(4), pp. 155–182, November 20, 2020 Deep-water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada Vinay P. Padate1, 2, Shivam Tiwari1, 3, Sherine Sonia Cubelio1,4 and Masatsune Takeda5 1Centre for Marine Living Resources and Ecology, Ministry of Earth Sciences, Government of India. Atal Bhavan, LNG Terminus Road, Puthuvype, Kochi 682508, India 2Corresponding author: [email protected]; https://orcid.org/0000-0002-2244-8338 [email protected]; https://orcid.org/0000-0001-6194-8960 [email protected]; http://orcid.org/0000-0002-2960-7055 5Department of Zoology, National Museum of Nature and Science, Tokyo. 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan. [email protected]; https://orcid/org/0000-0002-0028-1397 (Received 13 August 2020; accepted 23 September 2020) Abstract Deep-water squat lobsters collected during five cruises of the Fishery Oceanographic Research Vessel Sagar Sampada off the Andaman and Nicobar Archipelagos (299–812 m deep) and three cruises in the southeastern Arabian Sea (610–957 m deep) are identified. They are referred to each one species of the families Chirostylidae and Sternostylidae in the Superfamily Chirostyloidea, and five species of the family Munidopsidae and three species of the family Muni- didae in the Superfamily Galatheoidea. Of altogether 10 species of 5 genera dealt herein, the Uro- ptychus species of the Chirostylidae is described as new to science, and Agononida aff. indocerta Poore and Andreakis, 2012, of the Munididae, previously reported from Western Australia and Papua New Guinea, is newly recorded from Indian waters. -

Crustacea: Decapoda: Anomura: Chirostylidae) from Taiwan
Zootaxa 1919: 1–24 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Five new species of chirostylid crustaceans (Crustacea: Decapoda: Anomura: Chirostylidae) from Taiwan KEIJI BABA1 & CHIA-WEI LIN2 1Kumamoto University, Faculty of Education, 2-40-1 Kurokami, Kumamoto 860-8555, Japan. E-mai: [email protected] 2Crustacean Laboratory, Institute of Marine Biology, National Taiwan Ocean University, 2 Pei-Ning Rd, Keelung 20224, Taiwan. E-mail: [email protected] Abstract Five new species of chirostylid crustaceans from Taiwan are described. Eumunida chani sp. nov. is characterized by the absence of a pad of densely-packed setae on the pereopod 1, the anterior branchial margin bearing two spines, and the carpus of first pereopod with only two spines. Uroptychus anacaena sp. nov. and U. anatonus sp. nov. are similar but separated from each other by the shape of sternite 4 and relative length of the antennal scale. These species closely resemble U. maori Borradaile, 1916 and U. brucei Baba, 1986, but lack a ventral subterminal spine on the ischium of pereopod 1. Uroptychus orientalis sp. nov. is separated from U. occidentalis Faxon, 1893 by the shorter antennal scale and P2–4 dactyli with ultimate and penultimate spines subequal in size. Uroptychus singularis sp. nov. is distinguished from U. australis (Henderson, 1885) by the single, unpaired terminal spine on the flexor margin of pereopods 2–4. Key words: chirostylid crustaceans Introduction This paper is a part of a series of projects on the crustacean fauna of Taiwan (Tin-Yam Chan, project leader). -

A Novel Interaction: the Thin Stripe Hermit Crab, Clibanarius
A NOVEL INTERACTION: THE THIN STRIPE HERMIT CRAB, CLIBANARIUS VITTATUS, KILLS THE FLORIDA CROWN CONCH, MELONGENA CORONA, FOR ITS SHELL by Jennifer Cutter A Thesis Submitted to the Faculty of Charles E. Schmidt College of Science In Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, FL August 2017 Copyright by Jennifer Cutter 2017 ii ACKNOWLEDGEMENTS I would like to thank Florida Atlantic University, Harbor Branch Oceanographic Institute, and Dr. Donna Devlin for giving me the opportunity to conduct this fascinating study. I would also like to thank the other committee members (Dr. Vincent Encomio, Dr. Edward Proffitt, and Dr. William Brooks) for their help, advice, and guidance. This work was made possible through funding from the Indian River Lagoon Research Fellowship awarded by the Harbor Branch Foundation and a scholarship awarded by The Broward Shell Club. Additionally, I would like to thank Dr. Richard Turner for being willing to meet with me on several occasions to answer questions and share his vast knowledge. iv ABSTRACT Author: Jennifer Cutter Title: A Novel Interaction: The thin stripe hermit Crab, Clibanarius vittatus, kills the Florida crown conch, Melongena corona, for its shell Institution: Florida Atlantic University Thesis Advisor: Dr. Donna Devlin Degree: Master of Science Year: 2017 The hermit crab Clibanarius vittatus kills Melongena corona solely to acquire a better fitting shell. This finding is contrary to previous studies, which found that hermit crabs of other species cannot kill gastropods or, in most instances, remove freshly dead gastropods from their shells. This interaction cannot be classified as predation because Melongena tissue was never consumed. -

Chirostylid and Galatheid Crustaceans of Madagascar (Decapoda, Anomura) by Keiji BABA
CW'^T/' ' ' ' • "'W Sivli!i ... ^ 11TUTI0N RETURN TO W-119 Bull. Mus. natn. Hist, nat., Paris, 4e ser., 11, 1989, section A, n° 4 : 921-975. Chirostylid and Galatheid Crustaceans of Madagascar (Decapoda, Anomura) by Keiji BABA Bull. Mus. natn. Hist, nat., Paris, 4e ser., 11, 1989, section A, n° 4 : 921-975. Chirostylid and Galatheid Crustaceans of Madagascar (Decapoda, Anomura) by Keiji BAB A Abstract. — The chirostylid and galatheid crustaceans from Madagascar and vicinity in the collection of the Museum national d'Histoire naturelle, Paris, comprise 37 species (16 of Chirostylidae and 21 of Galatheidae) including nine new species : Eumunida bispinata, E. similior, Uroptychus brevipes, U. crassior, U. crosnieri, U. longioculus, Galathea anepipoda, G. robusta, and Munida remota. Seventeen species are recorded for the first time from the western Indian Ocean. Twenty-four of the 38 Madagascan species occur in the Western Pacific. The chirostylid Uroptychus granulatus Benedict, 1902, previously known from only the Galapagos Islands, is recorded from Madagascar. The galatheids Sadayoshia miyakei Baba, 1969, and S. acroporae Baba, 1972, are synonymized with S. edwardsii (Miers, 1884) and Liogalathea imperialis (Miyake and Baba, 1967), is synonymized with L. laeviroslris (Balss, 1913). A key to families, genera and species of the Madagascan chirostylids and galatheids is provided. Resume. L'etude des Chirostylidae et des Galatheidae recoltes a Madagascar et dans les lies avoisinantes, conserves au Museum national d'Histoire naturelle, a permis d'identifier 37 especes (16 appartenant aux Chirostylidae et 21 aux Galatheidae). Neuf de ces especes sont nouvelles pour la science : Eumunida bispinata, E. similior, Uroptychus brevipes, U. -

With Description of Six New Species from the Kermadec Islands
Zoological Journal of the Linnean Society, 2009, 155, 542–582. With 18 figures A review of the New Zealand Chirostylidae (Anomura: Galatheoidea) with description of six new species from the Kermadec Islands KAREEN E. SCHNABEL* Downloaded from https://academic.oup.com/zoolinnean/article/155/3/542/3820987 by guest on 24 September 2021 Marine Biodiversity & Biosecurity, National Institute of Water and Atmospheric Research, Private Bag 14 901, Kilbirnie, Wellington, New Zealand Received 2 October 2007; accepted for publication 14 January 2008 Prior to the present study, seven species of deep-sea Chirostylidae (‘squat lobsters’), were known from New Zealand: Gastroptychus novaezelandiae, Uroptychodes spinimarginatus, Uroptychus australis, Uroptychus maori, Uropty- chus novaezelandiae, Uroptychus politus, and Uroptychus tomentosus. All species are examined from type material and discussed, original illustrations supplemented, and new records provided where available. Uroptychus maori and Uroptychus novaezelandiae are re-described. The chirostylid fauna of the Kermadec Islands, a remote group of islands north-east of New Zealand, is studied. Uroptychus alcocki and Uroptychus scambus are reported for the first time from New Zealand, and six new species of the genus Uroptychus are described. Distributional patterns of New Zealand species are discussed and a key to New Zealand Uroptychus species is presented. © 2009 The Linnean Society of London, Zoological Journal of the Linnean Society, 2009, 155, 542–582. ADDITIONAL KEYWORDS: Gastroptychus – south-western