Research.Pdf (960.2Kb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OTC Bulletin,30 July 2010, Page 1)
OTC16-12-10p1.qxd 14/12/10 06:35 Page 1 16 December 2010 COMPANY NEWS 3 Reckitt Benckiser set to buy Sinclair signs Decapinol 3 partner in US 36.6 resurrects its 4 India’s Paras for INR32.6bn expansion strategy Navamedic secures rights 5 to NYDA in three markets eckitt Benckiser is set to boost its con- GNC quiet on takeover 5 Rsumer healthcare business by acquiring speculation India’s Paras Pharmaceuticals for INR32.6 Mag-Ox buy fails to offset 6 billion (C546 million). OTC sales drop at Hi-Tech The premium price tag represents more than Beiersdorf to cut offering 7 eight-times Paras’ sales of INR4.0 billion in the as it focuses on skincare year to March 2010, and nearly 30-times the company’s operating earnings before interest, GENERAL NEWS 8 tax, depreciation and amortisation (EBITDA) of INR1.1 billion. McNeil recalls Rolaids 8 in Canada and the US The deal comes soon after Reckitt Benck- iser expanded its consumer healthcare business Buying Paras will give Reckitt Benckiser a portfolio of Three pharmacy bodies 9 OTC brands in India including Moov topical analgesics speak with single voice with the purchase of SSL International and its Durex and Scholl brands for £2.54 billion (C3.02 Paras’ personal-care business, meanwhile, ASA gives Lyclear 11 the all-clear in UK billion) (OTC bulletin,30 July 2010, page 1). was led by the Set Wet brand of hair gels and Commenting on the Paras deal, Bart Becht, deodorants for men, the company said. MARKETING NEWS 12 Reckitt Benckiser’s chief executive officer,said The deal will also give Reckitt Benckiser a the growth potential of the business, the cre- new state-of-the-art and Good Manufacturing Clarityn’s fast acting claim 12 ation of a material healthcare business in India’s Practice compliant manufacturing plant in Nor- is stopped in UK by MHRA large and growing healthcare market, and the thern India, which employs around 700 people. -
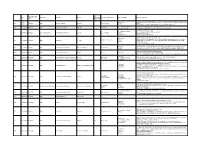
Category of Supplement Types of Claims Examples of Claims (2007) Enforceme Nt? •Germs Are Everywhere
Referred to Gov Date of Decision Type Challenger Advertiser Product Agency for Category of Supplement Types of Claims Examples of Claims (2007) Enforceme nt? •Germs are everywhere. Take Airborne to boost your immune system, fight viruses and help you stay Case •Performance 4648 3/26/2007 NAD Airborne Health Inc. Airborne Immune Health healthy. Report •Implied •Take Airborne. The immune boosting tablet that helps your body fight germs. •Clinically Proven/Shown •CH-Alpha is scientifically proven to promote joint health. 4652 Case Report 4/10/2007 NAD Gelita Health Products CH-Alpha Joint Health •Preventative Health •After just two to three months, you will regain the freedom of flexibility. •94% faster recovery [from colds] •Clinically Proven/Shown •Increased immune system resistance by 312% 4653 Case Report 4/10/2007 Proctor and Gamble Iovate Health Sciences Inc. Cold MD Immune Health •Speed •Clinically Proven results •Comparative •Doctor formulated and approved •Chromax helps your insulin function at its best. •Performance •It's an advanced, highly absorbable form of chromium that provides your body with the chromium it Blood Sugar Health •Insulin 4547 Compliance 4/11/2007 NAD Nutrition 21 Chromax needs to help promote healthy blood sugar, fight carbohydrate cravings and support your overall •Implied cardiovascular health. •Essential for optimum insulin health. •Exclusivity •One-A-Day Women's Multi-Vitamin is the only complete multi-vitamin with more calcium for strong 4672 Case Report 5/1/2007 NAD Bayer Consumer Healthcare One-A-Day Women's Multi-Vitamin •Implied bones, and now more vitamin D, which emerging research suggests may support breast cancer. -

Novartis Set to Introduce Pantoloc in 14 Countries
OTC10-02-10p1FIN.qxd 8/2/10 07:43 Page 1 10 February 2010 COMPANY NEWS 2 Novartis set to introduce Switzerland’s Mepha aims 2 at herbals and supplements Alliance Boots to establish 3 Swedish pharmacy venture Pantoloc in 14 countries Ta isho suffers as OTC market cools 4 J&J hit by comparison and currency 6 ovartis Consumer Health is set to in- the product is available without a prescription Ntroduce Nycomed’s proton-pump in- in Australia. NBTY’s pre-tax profits soar 7 hibitor Pantoloc Control as a non-prescrip- Novartis said that it would introduce Panto- Demand for OTC specialties 8 tion medicine in 14 European countries. loc Control in the 14 countries “later in 2010” lifts annual sales at Boiron The 20mg pantoprazole tablets gained non- through a co-marketing agreement with Nyco- Prilosec OTC hit by Prevacid 9 prescription status across Europe in June of last med. “The launch will be backed by substan- MiraLAX rival drives Perrigo 10 year through the European Union’s centralis- tial investment to build consumer awareness Omega offsets tough 11 ed authorisation procedure (OTC bulletin,19 and demand,”the company commented, “as start to grow sales June 2009, page 1). well as pharmacy training to help pharmacy Cadbury’s shareholders agree 12 Nycomed has since introduced the medi- teams identify customers who will benefit most to Kraft takeover cine for frequent heartburn in Austria, Estonia, from Pantoloc Control and advise those cus- William Ransom agrees to 13 Finland, France, Germany, Hungary,Latvia, tomers appropriately.” divest Manuka Gold Lithuania, the Netherlands,Norway,Poland, Brian McNamara, head of Europe for Nov- GENERAL NEWS 14 Sweden and Switzerland. -

Office Results
Raymond Wayne Whitted MD, MPH, FACOG …dedicated to safe, state-of-the-art surgery and health life-styles for women of all ages …because quality is an experience! www.drwhitted.net The Skinny on Weight Loss Supplements: Fact or Fantasy? Introduction Nowadays, it seems as though most Americans are trying to lose weight, and for good reason: about 97 million Americans are overweight or obese.[1] These patients have increased risk of all-cause mortality, as well as increased morbidity from hypertension, dyslipidemia, type 2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, and other respiratory problems, as well as certain malignancies, such as cancers of the endometrium, prostate, and breast.[1] The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Disease published guidelines for the treatment of overweight and obese adults.[1] These recommendations are intended for patients with a body mass index (BMI) ≥ 30, or ≥ 27 with obesity-related risk factors or diseases present. The guidelines recommend initial lifestyle modifications, including a reduced-calorie diet of 500-1000 calories per day, increased physical activity, and behavioral therapy. If these changes fail to produce a result in 6 months, approved pharmacotherapy is recommended as an adjunct in high-risk patients. Prescription medications approved by the US Food and Drug Administration (FDA) for weight loss include: sibutramine (Meridia, by Abbott Laboratories), which inhibits the reuptake of serotonin, norepinephrine, and dopamine; orlistat (Xenical, by Roche Laboratories Inc.), a reversible inhibitor of gastric and pancreatic lipase; and phentermine, an adrenergic medication.[2] Less commonly prescribed drugs include diethylpropion, benzphetamine, and phendimetrazine. -

Handbook of PREBIOTICS and PROBIOTICS INGREDIENTS Health Benefits and Food Applications
Handbook of PREBIOTICS AND PROBIOTICS INGREDIENTS Health Benefits and Food Applications Handbook of PREBIOTICS AND PROBIOTICS INGREDIENTS Health Benefits and Food Applications Edited by SUSAN SUNGSOO CHO E. TERRY FINOCCHIARO Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2010 by Taylor and Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number: 978-1-4200-6213-7 (Hardback) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmit- ted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information storage or retrieval system, without written permission from the publishers. -

Brasil-Ingredientes-Trends-2020.Pdf
Ingredients Campinas SP 1ª Edição Ficha catalográfica elaborada pela Área de Documentação e Informação do Centro deTecnologia de Embalagem – CETEA Brasil ingredients trends 2020 [recurso eletrônico] / editores, Airton Vialta, Raul Amaral Rego – 1. ed. – Campinas : ITAL, 2014. 389 p. : il. ; 27 cm. ISBN 978-85-7029-124-0 1. Ingredientes alimentícios. 2. Alimentos processados. 3. Suplementos alimentares. I. Vialta, Airton. II. Garcia, Aline de Oliveira. III. Lemos, Ana Lúcia da Silva Correa. IV. Van Dender, Ariene Gimenes Fernandes. V. Bucione, Ary. VI. Gallina, Darlila Aparecida. VII. Marasca, Elza Teresinha Grael. VIII. Lajolo, Franco. IX. Alvim, Izabela Dutra. X. Moreno, Izildinha. XI. Madi, Luis. XII. Soler, Márcia. XIII. Berto, Maria Isabel. XIV. Silva, Marta Gomes da. XV. Zacarchenco, Patrícia Blumer. XVI. Rego, Raul Amaral. XVII. Instituto de Tecnologia de Alimentos. XVIII. Título. PRO IA - I M B X R E A - S T IL IA FI AN GOVERNO DO ESTADO DE SÃO PAULO Governador: Geraldo Alckmin SECRETARIA DE AGRICULTURA E ABASTECIMENTO SAA Secretária: Mônika Bergamaschi SecretárioAdjunto: Alberto José Macedo Filho Chefe de Gabinete: Silvio Manginelli AGÊNCIA PAULISTA DE TECNOLOGIA DOS AGRONEGÓCIOS APTA Coordenador: Orlando Melo de Castro INSTITUTO DE TECNOLOGIA DE ALIMENTOS – ITAL DiretorGeral: Luis Madi Instituto de Tecnologia de Alimentos – ITAL Av. Brasil, 2.880 CEP: 13070178 – CampinasSP www.ital.sp.gov.br Criado em 30 de agosto de 1963, pelo Governo do Estado de A dinâmica do setor de ingredientes demonstra claramente que São Paulo, com o objetivo de auxiliar o desenvolvimento o segmento de alimentação e nutrição caminha de forma científico e tecnológico do setor industrial de alimentos, acelerada na direção de estágios avançados de tecnologia bebidas e embalagens, o ITAL adaptou-se, nesses 50 anos, embarcada. -

Healing*Edge Sciences
Extended Catalog Healing*Edge Science 102 Super Horny Goat Weed - 60 caps $12.50 4 EVER FIT 5219 Turbo Energy - 60 tabs $8.50 ID Description Price 2758 Yohimbe Power Max 2000 - 100 caps $20.50 6382 4Ever Whey Isolate Gainer Chocolate Shake - 8 $51.25 lbs 5646 Yohimbe Power Max 2000 - 2 fl.oz $14.25 6352 4Ever Whey Isolate Gainer Vanilla Shake - 8 lbs $51.25 AIRBORNE 6354 4Ever Whey Protein Chocolate Shake - 4 lbs $32.50 8825 4Ever Whey Protein Vanilla Shake - 4 lbs $32.50 ID Description Price 8308 Creatine Monohydrate - 500 gr $11.95 9017 Airborne Lemon-Lime - 30 tabs $17.50 7457 F4rmula Lean Muscle Meal Chocolate - 2 lbs $25.98 8171 Airborne Lemon-Lime - 36 tabs $17.50 7455 F4rmula Lean Muscle Meal Cookies & Cream - 2 $25.98 8529 Airborne Pink Grapefruit - 30 tabs $17.50 lbs 8659 Airborne Zesty Orange - 30 tabs $17.50 7456 F4rmula Lean Muscle Meal Vanilla - 2 lbs $25.98 9127 Airborne Zesty Orange - 30 tabs $17.50 6383 Fruit Blast the Isolate Tropical Mango - 5 lbs $59.95 7204 Fruit Blast the Natural Tropical Splash - 2 lbs $30.49 AJINOMOTO 6452 Fruit Blast the Whey Cranrazz - 5 lbs $32.50 ID Description Price 8826 Fruit Blast the Whey Mango Magic - 4 lbs $32.50 8311 Amino Vital 7500 Recovery and Energy ER Arctic $37.05 Lemon - 16 oz 9393 Fruit Blast the Whey Strawberry Kiwi - 4 lbs $32.50 9466 Amino Vital 7500 Recovery and Energy ER Fuji $37.05 6394 Fruit Blast the Whey Tangy Orange - 5 lbs $29.95 Apple - 16 oz 6471 L-Glutamine - 1000 gr $33.95 7196 Amino Vital Conditioning - 90 caps $18.90 6395 L-Glutamine - 500 gr $20.98 7352 Amino -

NBJ's Supplement Business Report
NBJ’s Supplement Business 2011 Report An analysis of markets, trends, competition and strategy in the U.S. dietary supplement industry NBJ’s Supplement Business Report 2011 2 www.nutritionbusinessjournal.com ©2011 Penton Media, Inc. Table of Contents 2.1. ACKNOWLEDGEMENTS ................................................................................................................................................... 23 2.2. RESEARCH METHODOLOGY ............................................................................................................................................. 23 2.2.1. Disclaimer ........................................................................................................................................................ 24 2.2.2. Copyright ......................................................................................................................................................... 24 2.3. DEFINITIONS ................................................................................................................................................................. 24 2.3.1. Product Categories .......................................................................................................................................... 24 2.3.2. Sales Channels ................................................................................................................................................. 26 3.1. U.S. NUTRITION INDUSTRY ............................................................................................................................................