Novartis Set to Introduce Pantoloc in 14 Countries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reckitt Benckiser 0000 Paracetamol Posology Update 2019-04
Reckitt Benckiser 0000 Paracetamol Posology Update 2019-04 Package Leaflet: Information for the User • Sodium (129.0mg per sachet), main component of cooking/table salt. This is equivalent to 6.45% of RB Artwork and Lemsip Max Honey & Ginger Flavour Powder for Oral Solution the recommended maximum daily dietary intake of sodium for an adult. Print Specification (paracetamol and phenylephrine hydrochloride) 3. How to take this medicine Trident Reference No: RB251338 Please read this leaflet carefully before you take this medicine. If you are not sure of anything ask your To be made into a hot drink. Dissolve one sachet in a mug of hot but not boiling water. Stir until dissolved. If preferred sweeten to taste with sugar, honey or your usual sweetener. ZEN Ref: TR1197000 pharmacist or doctor. 250 It is important to drink plenty of fluids when suffering from colds and flu. Action: D 1. What this medicine is and what it is used for? Adults, the elderly and children 16 years and over: Brand: Lemsip Lemsip Max Honey & Ginger Flavour Powder for Oral Solution contains a combination of ingredients One sachet every 4-6 hours as required. Do not take more than 4 sachets in 24 hours. Category: Max which are effective in relieving the symptoms associated with colds and flu, including relief of aches and Segment Group: Cold & Flu pains, sore throats, headache, nasal congestion and lowering of temperature. Do not give to children under 16 years. Segment: Honey & Ginger Paracetamol is a well-known painkiller (analgesic). It is effective against aches and pains, including a headache, If the symptoms of your cold or flu persist for more than three days, or worsen, consult your pharmacist. -

Betterbusiness Betterfinancials How We Drive Growth and Outperformance
Reckitt Benckiser Group plc Annual Report and Financial2015 Statements betterbusiness 2015 Reckitt Benckiser Group plc (RB) Annual Report and Financial Statements We make a difference to people’s lives through a trusted portfolio of brands, across consumer health, hygiene and home. Our vision Our purpose A world where people are To make a difference, by healthier and live better. giving people innovative solutions for healthier lives and happier homes. Our strategy betterbusiness betterfinancials How we drive growth and outperformance Chief Executive’s Review on pages 8–9 bettersociety betterenvironment How we support How we reduce our communities and our environmental develop our people impact Strategic framework on pages 12–13 Contents Strategic Report bettersociety Governance Report 1 Highlights 24 – Workplace 46 Board of Directors 2 At a glance 26 – Communities 50 Executive Committee 4 Chairman’s Statement 26 – Products 52 Chairman’s Statement on 7 Reasons why RB delivers betterenvironment Corporate Governance 8 Chief Executive’s Review 27 – Greenhouse gas emissions 54 Corporate Governance Statement 10 Our unique culture 28 – Water 60 Nomination Committee Report 12 Strategic framework 28 – Waste 61 Audit Committee Report 14 Our market and resources 29 – Sourcing 66 Directors’ Remuneration Report betterfinancials 30 Our operating model 68 Our remuneration at a glance 16 – Our strategy to deliver 32 Our operating model in action 70 Annual Report on Remuneration 17 – Organisation 34 Creating stakeholder value 79 Directors’ Remuneration Policy 19 – Powermarkets 36 Financial Review 85 Report of the Directors 20 – Powerbrands 40 Strategic Risks 88 Directors’ Statement of Responsibilities 22 – Virtuous earnings model Financial Statements 89 Financial Statements Any information contained in the 2015 Annual Report and Financial Statements on the price at which shares or other securities in Reckitt Benckiser Group plc have been bought or sold in the past, or on the yield on such shares or other securities, should not be relied upon as a guide to future performance. -

Rejuvenating RB Plc Group Benckiser Reckitt Annual Report and Financial Statements 2019 Statements Financial and Report Annual
Rejuvenating RB Reckitt Benckiser Group plc Annual Report and FinancialStatements 2019 Reckitt Benckiser Group plc Annual Report and Financial Statements 20192018 INTRODUCTION CONTENTS Strategic Report Welcome 01 Financial highlights 02 Health and Hygiene Home – at a glance 04 Chairman’s statement 06 Chief Executive’s statement 10 Our business model Our purpose 12 How purpose drives our performance We exist to protect, heal and nurture in the relentless pursuit of a cleaner 14 Mapping what matters to our stakeholders and healthier world. 18 KPIs 20 Consumers Our fight 24 Customers We have a fight on our hands. A fight to make access to the highest quality 28 People hygiene, wellness and nourishment a right and not a privilege. 32 Partners 36 Communities 40 Environment 46 s172 statement 48 Health operating review 52 Hygiene Home operating review 56 Non-financial information statement 58 Financial review 64 Risk management Health 77 Viability statement Governance 78 Board of Directors 84 Executive Committee 86 Corporate Governance – Chairman’s statement 88 Corporate Governance statement 97 Nomination Committee Report 103 Audit Committee Report 111 CRSEC Committee Report 117 Directors’ Remuneration Report 138 Report of the Directors Page 48 141 Statement of Directors’ Responsibilities Financial Statements 143 Independent Auditors Report Chief Executive’s Statement 152 Financial Statements 223 Shareholder Information Hygiene Home Page 06 Page 52 STRATEGIC REPORT GOVERNANCE FINANCIAL STATEMENTS FINANCIAL HIGHLIGHTS Net Revenue Health Hygiene -

OTC Bulletin,30 July 2010, Page 1)
OTC16-12-10p1.qxd 14/12/10 06:35 Page 1 16 December 2010 COMPANY NEWS 3 Reckitt Benckiser set to buy Sinclair signs Decapinol 3 partner in US 36.6 resurrects its 4 India’s Paras for INR32.6bn expansion strategy Navamedic secures rights 5 to NYDA in three markets eckitt Benckiser is set to boost its con- GNC quiet on takeover 5 Rsumer healthcare business by acquiring speculation India’s Paras Pharmaceuticals for INR32.6 Mag-Ox buy fails to offset 6 billion (C546 million). OTC sales drop at Hi-Tech The premium price tag represents more than Beiersdorf to cut offering 7 eight-times Paras’ sales of INR4.0 billion in the as it focuses on skincare year to March 2010, and nearly 30-times the company’s operating earnings before interest, GENERAL NEWS 8 tax, depreciation and amortisation (EBITDA) of INR1.1 billion. McNeil recalls Rolaids 8 in Canada and the US The deal comes soon after Reckitt Benck- iser expanded its consumer healthcare business Buying Paras will give Reckitt Benckiser a portfolio of Three pharmacy bodies 9 OTC brands in India including Moov topical analgesics speak with single voice with the purchase of SSL International and its Durex and Scholl brands for £2.54 billion (C3.02 Paras’ personal-care business, meanwhile, ASA gives Lyclear 11 the all-clear in UK billion) (OTC bulletin,30 July 2010, page 1). was led by the Set Wet brand of hair gels and Commenting on the Paras deal, Bart Becht, deodorants for men, the company said. MARKETING NEWS 12 Reckitt Benckiser’s chief executive officer,said The deal will also give Reckitt Benckiser a the growth potential of the business, the cre- new state-of-the-art and Good Manufacturing Clarityn’s fast acting claim 12 ation of a material healthcare business in India’s Practice compliant manufacturing plant in Nor- is stopped in UK by MHRA large and growing healthcare market, and the thern India, which employs around 700 people. -

MARKETING FARMACEUTICO (Farmaco, Parafarmaco E Otc)
Percorso di lettura a cura di Largo Consumo Rivista di economia a marketing sulla filiera dei beni di consumo www.largoconsumo.info Documento in versione interattiva: www.largoconsumo.info/112006/PL-1106-009.Pdf MARKETING FARMACEUTICO (Farmaco, Parafarmaco e Otc) Costi del Percorso di lettura: Per ordini: [email protected] € 178,50 (comprensivo iva) - non abbonati Aggiornato a: Largo Consumo 11/2006 e supplementi € 159,00 (comprensivo iva) - abbonati Rif: PL-1106-009 Costi dei singoli titoli: http://www.largoconsumo.info/Largoconsumo/fixed/largoconsumo/pagine/diffusione.asp#Costi RIFERIMENTI: Il sommario di tutti i Percorsi di lettura di Largo Consumo è scaricabile all’indirizzo WWW.LARGOCONSUMO.INFO/PERCORSI LE FONTI DI QUESTO PERCORSO DI LETTURA E SUGGERIMENTI PER L’APPROFONDIMENTO DEI TEMI: Largo Consumo OSSERVATORIO Rivista di economia e marketing sulla filiera dei beni di consumo D’IMPRESA: Mensile fondato nel 1980 e diffuso esclusivamente in abbonamento, i cui contenuti giornalistici si sviluppano in forma di inchieste, studi e articoli vari inerenti tutti i Leggi le case history momenti della filiera dei beni mass market, food e non food., dalla produzione, alla di COMUNICAZIONI distribuzione, al consumo finale, compresi i servizi, le strutture e i sistemi collegati. D’IMPRESA di Aziende e Mercato & Imprese Organismi attivi nei Opinioni e prospettive dall’industria del largo consumo mercati considerati Ogni anno, Mercato & Imprese ospita interviste ad alcuni fra i più accreditati in questo Percorso di esponenti di aziende di primo piano dell’industria alimentare e grocery non food. I lettura selezionati contenuti giornalistici sono sviluppati in forma di interviste, tante quanti sono i da Largo Consumo settori merceologici presi in esame nel fascicolo. -

Solid Dosage Drug Development and Manufacturing
SUPPLEMENT TO THE APRIL 2020 ISSUE OF 2020 PharmTech.com Solid Dosage Drug Development and Manufacturing Development OTC Formulations Quality Excipient Regulations Manufacturing Tableting Factors Multi-Tip Tooling Operations OSD Production Challenges CONTRACT MANUFACTURING Biologics | ADCs | Potent | Drug Product | Fermentation Fill/Finish | Hot Melt Extrusion | APIs abbviecontractmfg.com Experience Drug Product Unrivaled When it comes to delivering drug product, you need a CMO with longstanding commercial expertise to ensure fast time to market and assurance of supply. Solid Dosage Drug Development and Manufacturing 2020 EDITORIAL DEVELOPMENT Editorial Director Rita Peters [email protected] Senior Editor Agnes Shanley [email protected] Managing Editor Susan Haigney [email protected] s4 Oral Dosage Form Innovation European Editor Felicity Thomas [email protected] in OTC Pharmaceuticals Manufacturing Editor Jennifer Markarian [email protected] Science Editor Feliza Mirasol [email protected] Gerry McNally Assistant Editor Lauren Lavelle [email protected] Senior Art Director Marie Maresco; Graphic Designer Maria Reyes Contributing Editors Jill Wechsler [email protected]; Hallie Forcinio [email protected]; QUALITY/REGULATIONS Susan J. Schniepp [email protected]; Eric Langer [email protected]; and Cynthia A. Challener, PhD [email protected] s10 Considering Excipient Regulations Correspondent Sean Milmo (Europe, [email protected]) 485 Route One -

OTC Bulletin,10 Septem- with New Names in the UK Through the Acquisition, the Swedish Com- Ber 2010, Page 1)
OTC16-11-10p1FIN.qxd 15/11/10 07:02 Page 1 16 November 2010 COMPANY NEWS 3 Sanofi expands in China Alliance Boots eyes 3 Chinese expansion Regulatory problems hit 4 Mucinex launch with BMP Sunstone deal Reckitt plans future 5 of Durex and Scholl Merck overcomes 6 anofi-Aventis is set to strengthen its pres- der exclusive multi-year licenses and provides Latin American drop Sence in the Chinese OTC market by ac- pharmaceutical distribution services through Sanofi-Aventis works on 7 quiring BMP Sunstone and its portfolio of subsidiaries in Beijing and Shanghai. consumer acquisitions OTC cough/cold and women’s health brands Sanofi-Aventis said the US$10.00 per share Energy drinks boost 8 for around US$520 million (C375 million). deal had been unanimously approved by BMP OTC sales at Taisho The price tag represents around 3.5-times Sunstone’s board of directors and had received Prilosec OTC holds back 9 BMP Sunstone’s 2009 sales of US$147 million the backing of shareholders representing 23% growth at P&G and 62-times its operating profit of US$8.4 mil- of the company’s stock. Perrigo sees longevity 10 lion. The company is based in the US and oper- Combining BMP Sunstone with its exist- in store-brands ates through subsidiaries in China. ing Chinese joint venture business, Hangzhou Pfizer benefits from 12 Almost 60% of BMP Sunstone’s sales were Sanofi Minsheng Consumer Healthcare, would Wyeth’s OTC brands generated by its OTC brands, Sanofi-Aventis turn Sanofi-Aventis into a “leading consumer Key brands boost 13 said, which included China’s leading paediatric healthcare company in China”, the French firm Bayer Consumer Care cough and cold product Hao Wa Wa (Good- said, with a strong position in China’s two largest Merck eyes global future 15 Baby) and the women’s hygiene brand Kang OTC categories of cough and cold and vitamin for Consumer Care division Fu Te (Confort). -

Statistical Analysis Plan
Non-Interventional Study Protocol Study Code << DXXXRXXX >> Version V1.4 Date 14 July 2017 Decline In lung-function Among Patients with chronic obstructive Lung disease On maintenance therapy (DIAPLO) An observational study evaluating the benefits of early intervention with maintenance therapies to prevent or slow down rapid lung function decline in patients who are at high risk at the time of COPD diagnosis in the combined Optimum Patient Care Research Database and Clinical Practice Research Datalink databases TITLE PAGE Non-Interventional Study Protocol Study Code << DXXXRXXX >> Version 14 July 2017 Date 14 July 2017 TABLE OF CONTENTS PAGE TITLE PAGE ........................................................................................................... 1 TABLE OF CONTENTS ......................................................................................... 2 LIST OF ABBREVIATIONS .................................................................................. 5 RESPONSIBLE PARTIES ...................................................................................... 6 PROTOCOL SYNOPSIS DIAPLO STUDY ........................................................... 7 AMENDMENT HISTORY ................................................................................... 12 MILESTONES ....................................................................................................... 13 1. BACKGROUND AND RATIONALE .................................................................. 14 1.1 Background ........................................................................................................... -

Icontact Newsletter
eckitt Benckiser (formerly Reckitt & C sioners of the R olman) Pensio i for pen n Fund No. 69, September 2017 IN THIS ISSUE ROUTINE INCREASE LETTER When Hull’s Weeping Window poppy display moved on, Derby put its best foot forward with a pavement trail leading to a IS ALSO A REASSURANCE display reaching new heights The April letter, tailored to the individual and sent to RBPA at the Old Silk Mill (see P3&9). members advising them of the annual increase in their pension, may be routine but, like the annual update newsletter, is a welcome reassurance in these uncertain economic times. Also on P3 see how Dansom The letter notes: ‘The level of maintain the century-old tradition Lane’s British Legion standard annual increase applied to your Fund of employee welfare, to use the old- came out of retirement for the pension is equal to the year-on-year fashioned but very relevant words. “Poppy for Oppy” display change in the Retail Prices Index In both business and Government, (RPI) at the 31 December prior to the pensions have become a numbers increase. The rate at 31 December game. Deficits thwart takeover 2016 was 2.5%. Therefore, with effect bids; underfunding or retirement from 1 April 2017, your Fund pension age extensions could impoverish will be increased by 2.5%. some pensioners; and it is reported ‘This is in accordance with the that the government relaxation to Fund’s Rules, which provide that the allow pensioners to cash-in early is Trustees must increase pensions in resulting in serious personal debt line with RPI, up to a maximum of 5%.’ problems. -

Contact 77 to See the Results for the “Brand Search”That Was Published in the Last Magazine
No. 77 April 2020 RB donated £5.5 million to help combat GDPR & Pensioner Visiting resolved Covid-19 (Coronavirus) in China As you will know, we’ve had quite a In the early stages of the outbreak, RB to the moral responsibility we feel, we time trying to review our Pensioner immediately coordinated 600,000 also have an important functional role visiting procedures and updating RMB (about £60k) worth of soap and to play in enhancing personal them in order to comply with GDPR sanitizer products to help meet the disinfection through providing and meet the standards expected by cleaning and disinfection enhanced access to products which RB. We are now very pleased, and requirements in Wuhan’s hospitals. can break the chain of infection. not a little relieved, to say that the Commenting on the outbreak, RB Simple steps such as frequent hand process is now complete. CEO Laxman Narasimhan said: “We washing will aid the many efforts the immediately mobilised our experts in Chinese government is already *** STOP PRESS *** China and beyond as soon as the putting in place to protect citizens outbreak was identified. In addition across the region.” Due to the Corona Virus, we will (Press release: www.rb.com) not be making pensioner visits, in person, at the present time. All RB’s End of Year Results for 2019 other formats of Pensioner visiting RB Chief Executive, Laxman our communities. While the recent years - phone calls, WhatsApp messaging Narasimhan commented on the results have been difficult, I believe strongly in and emails - will remain in place. -
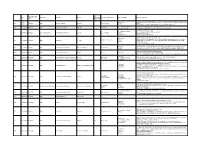
Category of Supplement Types of Claims Examples of Claims (2007) Enforceme Nt? •Germs Are Everywhere
Referred to Gov Date of Decision Type Challenger Advertiser Product Agency for Category of Supplement Types of Claims Examples of Claims (2007) Enforceme nt? •Germs are everywhere. Take Airborne to boost your immune system, fight viruses and help you stay Case •Performance 4648 3/26/2007 NAD Airborne Health Inc. Airborne Immune Health healthy. Report •Implied •Take Airborne. The immune boosting tablet that helps your body fight germs. •Clinically Proven/Shown •CH-Alpha is scientifically proven to promote joint health. 4652 Case Report 4/10/2007 NAD Gelita Health Products CH-Alpha Joint Health •Preventative Health •After just two to three months, you will regain the freedom of flexibility. •94% faster recovery [from colds] •Clinically Proven/Shown •Increased immune system resistance by 312% 4653 Case Report 4/10/2007 Proctor and Gamble Iovate Health Sciences Inc. Cold MD Immune Health •Speed •Clinically Proven results •Comparative •Doctor formulated and approved •Chromax helps your insulin function at its best. •Performance •It's an advanced, highly absorbable form of chromium that provides your body with the chromium it Blood Sugar Health •Insulin 4547 Compliance 4/11/2007 NAD Nutrition 21 Chromax needs to help promote healthy blood sugar, fight carbohydrate cravings and support your overall •Implied cardiovascular health. •Essential for optimum insulin health. •Exclusivity •One-A-Day Women's Multi-Vitamin is the only complete multi-vitamin with more calcium for strong 4672 Case Report 5/1/2007 NAD Bayer Consumer Healthcare One-A-Day Women's Multi-Vitamin •Implied bones, and now more vitamin D, which emerging research suggests may support breast cancer. -

Acquisition Agreement for SSL International -- Current Agreements
Dealdoc Acquisition agreement for SSL International Reckitt Benckiser SSL International Jul 21 2010 © 2009-2021, Wildwood Ventures Ltd. All rights reserved. Acquisition agreement for SSL International Reckitt Benckiser Companies: SSL International Announcement date: Jul 21 2010 Deal value, US$m: 3900.0 : transaction value • Details • Financials • Termsheet • Press Release • Filing Data • Contract Details Announcement date: Jul 21 2010 Industry sectors: Consumer health Financials Deal value, US$m: 3900.0 : transaction value Termsheet 21 July 2010 Reckitt Benckiser has agreed to buy Durex condom maker SSL International for 2.54 billion pounds. Press Release RECOMMENDED CASH OFFER FOR SSL INTERNATIONAL PLC BY RECKITT BENCKISER PLC, A WHOLLY-OWNED SUBSIDIARY OF RECKITT BENCKISER GROUP PLC Summary of the Offer The boards of Reckitt Benckiser Group plc ("Reckitt Benckiser") and SSL International plc ("SSL") are pleased to announce that they have reached agreement on the terms of a recommended cash offer to be made by Reckitt Benckiser plc, a wholly-owned subsidiary of Reckitt Benckiser, to acquire the entire issued and to be issued share capital of SSL (the “Offer”). SSL is a focused consumer products company with leading global brands such as Durex and Scholl, as well as a portfolio of local brands. Reckitt Benckiser is a world leader in household and health & personal care. The acquisition of SSL provides Reckitt Benckiser with an attractive opportunity to increase its presence in the health & personal care sector. Under the terms of the Offer, SSL Shareholders will be entitled to receive 1163 pence in cash per SSL Share (the “Offer Price”) and will also remain entitled to receive the proposed final dividend of 8 pence per share in respect of the year ended 31 March 2010 (the “SSL Dividend”), representing, in aggregate, 1171 pence per SSL Share.