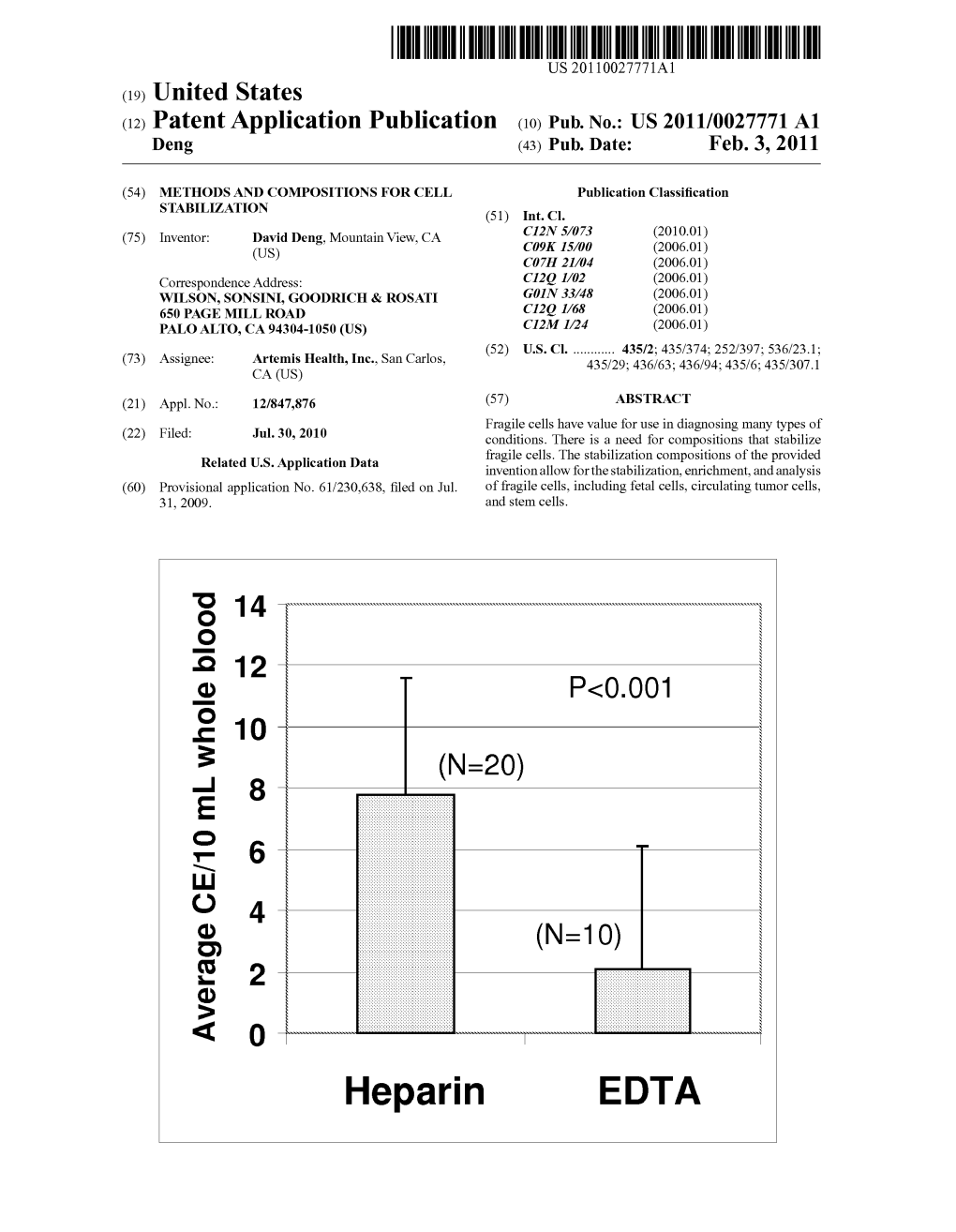
Heparin EDTA Patent Application Publication Feb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao Et Al
USOO9498481 B2 (12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao et al. (45) Date of Patent: *Nov. 22, 2016 (54) CYCLOPROPYL MODULATORS OF P2Y12 WO WO95/26325 10, 1995 RECEPTOR WO WO99/O5142 2, 1999 WO WOOO/34283 6, 2000 WO WO O1/92262 12/2001 (71) Applicant: Apharaceuticals. Inc., La WO WO O1/922.63 12/2001 olla, CA (US) WO WO 2011/O17108 2, 2011 (72) Inventors: Tadimeti Rao, San Diego, CA (US); Chengzhi Zhang, San Diego, CA (US) OTHER PUBLICATIONS Drugs of the Future 32(10), 845-853 (2007).* (73) Assignee: Auspex Pharmaceuticals, Inc., LaJolla, Tantry et al. in Expert Opin. Invest. Drugs (2007) 16(2):225-229.* CA (US) Wallentin et al. in the New England Journal of Medicine, 361 (11), 1045-1057 (2009).* (*) Notice: Subject to any disclaimer, the term of this Husted et al. in The European Heart Journal 27, 1038-1047 (2006).* patent is extended or adjusted under 35 Auspex in www.businesswire.com/news/home/20081023005201/ U.S.C. 154(b) by Od en/Auspex-Pharmaceuticals-Announces-Positive-Results-Clinical M YW- (b) by ayS. Study (published: Oct. 23, 2008).* This patent is Subject to a terminal dis- Concert In www.concertpharma. com/news/ claimer ConcertPresentsPreclinicalResultsNAMS.htm (published: Sep. 25. 2008).* Concert2 in Expert Rev. Anti Infect. Ther. 6(6), 782 (2008).* (21) Appl. No.: 14/977,056 Springthorpe et al. in Bioorganic & Medicinal Chemistry Letters 17. 6013-6018 (2007).* (22) Filed: Dec. 21, 2015 Leis et al. in Current Organic Chemistry 2, 131-144 (1998).* Angiolillo et al., Pharmacology of emerging novel platelet inhibi (65) Prior Publication Data tors, American Heart Journal, 2008, 156(2) Supp. -

Asymmetric Organocatalyzed Synthesis of Coumarin Derivatives
Asymmetric organocatalyzed synthesis of coumarin derivatives Natália M. Moreira‡, Lorena S. R. Martelli‡ and Arlene G. Corrêa* Review Open Access Address: Beilstein J. Org. Chem. 2021, 17, 1952–1980. Centre of Excellence for Research in Sustainable Chemistry, https://doi.org/10.3762/bjoc.17.128 Department of Chemistry, Federal University of São Carlos, 13565-905 São Carlos, SP – Brazil Received: 08 March 2021 Accepted: 21 July 2021 Email: Published: 03 August 2021 Arlene G. Corrêa* - [email protected] This article is part of the thematic issue "New advances in asymmetric * Corresponding author ‡ Equal contributors organocatalysis". Keywords: Guest Editor: R. Šebesta asymmetric synthesis; green chemistry; 2H-chromen-2-one; organocatalysis © 2021 Moreira et al.; licensee Beilstein-Institut. License and terms: see end of document. Abstract Coumarin derivatives are essential scaffolds in medicinal and synthetic chemistry. Compounds of this class have shown important activities, such as anticancer and antiparasitic, besides the commercially available drugs. These properties led to the development of efficient and greener synthetic methods to achieve the 2H-chromen-2-one core. In this context, the advances in asymmetric organocatalyzed synthesis of coumarin derivatives are discussed in this review, according to the mode of activation of the catalyst. Introduction Coumarins are important naturally occurring plant constituents and display a wide range of pharmacological and biological ac- tivities, such as anticancer [1], antibacterial [2], and -

Characterising the Risk of Major Bleeding in Patients With
EU PE&PV Research Network under the Framework Service Contract (nr. EMA/2015/27/PH) Study Protocol Characterising the risk of major bleeding in patients with Non-Valvular Atrial Fibrillation: non-interventional study of patients taking Direct Oral Anticoagulants in the EU Version 3.0 1 June 2018 EU PAS Register No: 16014 EMA/2015/27/PH EUPAS16014 Version 3.0 1 June 2018 1 TABLE OF CONTENTS 1 Title ........................................................................................................................................... 5 2 Marketing authorization holder ................................................................................................. 5 3 Responsible parties ................................................................................................................... 5 4 Abstract ..................................................................................................................................... 6 5 Amendments and updates ......................................................................................................... 7 6 Milestones ................................................................................................................................. 8 7 Rationale and background ......................................................................................................... 9 8 Research question and objectives .............................................................................................. 9 9 Research methods .................................................................................................................... -

Reactivity and Alkylating Potential of Some O-Heterocycles
Dpto. de Qu´ımica f´ısica Facultad de Ciencias Qu´ımicas PhD Thesis Chemical processes that can damage cellular DNA: Reactivity and alkylating potential of some O-heterocycles Supervisors: Author: Prf. Dr. Julio Casado Rafael Gomez´ Bombarelli Prf. Dr. Emilio Calle November 8, 2011 A Marta, Jose,´ Lali y Pepe en ningun´ orden en particular Departamento de Qu´ımica f´ısica Facultad de Ciencias Qu´ımicas The work reported here has been carried out in the Departamento de Qu´ımica f´ısica of the Universidad de Salamanca, under the advice of Prof. Dr. Julio Casado Linarejos and Prof. Dr. Emilio Calle Mart´ın. Rafael Gomez´ Bombarelli Emilio Calle Mart´ın Julio Casado Linarejos It’s been emotional. Big Chris Acknowledgements The author thanks the Spanish Ministerio de Educacion´ for a PhD fellowship (AP2006-01976) and financed research stays at the workgroups of Prof. Dr. Jose´ Rueff (Universidade Nova de Lisboa) and Prof. Dr. Franc¸ois Maurel (ITODYS, Paris-VII), who are also thanked for their hos- pitality. Financial support of the research reported in this work by the Ministerio de Ciencia e Innovacion´ (projects CTQ2010-18999, CTQ2007-63263, CTQ2004-05048), and the Junta de Castilla y Leon´ and European Regional Development Fund (project SA040A08) is also ac- knowledged. E. Bombarelli and J. Arenas are also thanked for their generous donation of CPU-time and resources. i Contents List of Figuresv List of Tables vii List of Schemes ix Abbreviations xi Preface xiii 1 Alkylating agents and the NBP Test: a review 1 1.1 Development of the method....................5 1.1.1 Early examples.......................5 1.1.2 The Epstein test.......................6 1.1.3 Biological samples......................7 1.1.4 Chromatography......................7 1.2 NBP as a DNA-model.......................8 1.2.1 Nucleophilicity of DNA....................8 1.2.2 Site selectivity...................... -

Regulation of Tissue Factor and Coagulation Activity;
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 341 Regulation of Tissue Factor and Coagulation Activity; Translation Studies with Focus on Platelet-Monocyte Aggregates and Patients with Acute Coronary Syndrome CHRISTINA CHRISTERSSON ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6206 UPPSALA ISBN 978-91-554-7177-4 2008 urn:nbn:se:uu:diva-8669 ! " #$ %& #' ( )* " +, - . . , / /, %&, 0 - * / 12, - . * (3" 1 ( . 1 / , 1 , 45#, &$ , , 67 8$&38#353$#$$35, " )"+ 9 . , - .2 . ! . " ! . ! . 2 3 )("1+, ! . " . : %53; , - . 3 ! )*#<% 3 + , ; = 3 .! , - . ! , 6 )-*+ , ! , - ("13 . 3 ", ("1, * ("1 . . (3 -* 3 > , 1 ! 3 " . . , 3 . ! . , / ("1 " , ? (3 -* > , " / (3 - - ! "#$%&'% @ / / %& 7 #;#3;%; 67 8$&38#353$#$$35 ' ''' 3&;;8 ) 'AA ,!,A B C ' ''' 3&;;8+ To my family and to science This thesis is based on the following papers I. Christersson C, Oldgren J, Bylock A, Wallentin L, Sieg- bahn A. Long-term treatment with ximelagatran, an oral di- rect thrombin inhibitor, persistently reduces the coagula- tion activity after a myocardial -

Desmodus Rotundus) Blood Feeding
toxins Article Vampire Venom: Vasodilatory Mechanisms of Vampire Bat (Desmodus rotundus) Blood Feeding Rahini Kakumanu 1, Wayne C. Hodgson 1, Ravina Ravi 1, Alejandro Alagon 2, Richard J. Harris 3 , Andreas Brust 4, Paul F. Alewood 4, Barbara K. Kemp-Harper 1,† and Bryan G. Fry 3,*,† 1 Department of Pharmacology, Biomedicine Discovery Institute, Faculty of Medicine, Nursing & Health Sciences, Monash University, Clayton, Victoria 3800, Australia; [email protected] (R.K.); [email protected] (W.C.H.); [email protected] (R.R.); [email protected] (B.K.K.-H.) 2 Departamento de Medicina Molecular y Bioprocesos, Instituto de Biotecnología, Universidad Nacional Autónoma de México, Av. Universidad 2001, Cuernavaca, Morelos 62210, Mexico; [email protected] 3 Venom Evolution Lab, School of Biological Sciences, University of Queensland, St. Lucia, Queensland 4067, Australia; [email protected] 4 Institute for Molecular Biosciences, University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (A.B.); [email protected] (P.F.A.) * Correspondence: [email protected] † Joint senior authors. Received: 20 November 2018; Accepted: 2 January 2019; Published: 8 January 2019 Abstract: Animals that specialise in blood feeding have particular challenges in obtaining their meal, whereby they impair blood hemostasis by promoting anticoagulation and vasodilation in order to facilitate feeding. These convergent selection pressures have been studied in a number of lineages, ranging from fleas to leeches. However, the vampire bat (Desmondus rotundus) is unstudied in regards to potential vasodilatory mechanisms of their feeding secretions (which are a type of venom). This is despite the intense investigations of their anticoagulant properties which have demonstrated that D. -

Coagulation Factors Directly Cleave SARS-Cov-2 Spike and Enhance Viral Entry
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.31.437960; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. Edward R. Kastenhuber1, Javier A. Jaimes2, Jared L. Johnson1, Marisa Mercadante1, Frauke Muecksch3, Yiska Weisblum3, Yaron Bram4, Robert E. Schwartz4,5, Gary R. Whittaker2 and Lewis C. Cantley1,* Affiliations 1. Meyer Cancer Center, Department of Medicine, Weill Cornell Medical College, New York, NY, USA. 2. Department of Microbiology and Immunology, Cornell University, Ithaca, New York, USA. 3. Laboratory of Retrovirology, The Rockefeller University, New York, NY, USA. 4. Division of Gastroenterology and Hepatology, Department of Medicine, Weill Cornell Medicine, New York, NY, USA. 5. Department of Physiology, Biophysics and Systems Biology, Weill Cornell Medicine, New York, NY, USA. *Correspondence: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2021.03.31.437960; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Summary Coagulopathy is recognized as a significant aspect of morbidity in COVID-19 patients. The clotting cascade is propagated by a series of proteases, including factor Xa and thrombin. Other host proteases, including TMPRSS2, are recognized to be important for cleavage activation of SARS-CoV-2 spike to promote viral entry. Using biochemical and cell-based assays, we demonstrate that factor Xa and thrombin can also directly cleave SARS-CoV-2 spike, enhancing viral entry. -

Clinical Trial Protocol Doc
Clinical Trial Protocol Doc. No.: c02214385-09 EudraCT No.: 2013 003201 26 BI Trial No.: 1160.186- - BI Investigational Pradaxa®, dabigatran etexilate Product(s): Title: A prospective Randomised, open label, blinded endpoint (PROBE) study to Evaluate DUAL antithrombotic therapy with dabigatran etexilate (110mg and 150mg b.i.d.) plus clopidogrel or ticagrelor vs. triple therapy strategy with warfarin (INR 2.0 – 3.0) plus clopidogrel or ticagrelor and aspirin in patients with non valvular atrial fibrillation (NVAF) that have undergone a percutaneous coronary intervention (PCI) with stenting (RE-DUAL PCI) Clinical Phase: IIIb Trial Clinical Monitor: Phone: Fax: Co-ordinating Investigator: Phone: Fax: Status: Final Protocol (Revised Protocol (based on global Amendments 1, 2 and 3)) Version and Date: Version: 4.0 Date: 21 July 2016 Page 1 of 150 Proprietary confidential information. 2016 Boehringer Ingelheim International GmbH or one or more of its affiliated companies. All rights reserved. This document may not - in full or in part - be passed on, reproduced, published or otherwise used without prior written permission. TITLE PAGE Boehringer Ingelheim 21 Jul 2016 BI Trial No.: 1160.186 Doc. No.: c02214385-09 Trial Protocol Version 4.0 Page 2 of 150 CLINICAL TRIAL PROTOCOL SYNOPSIS Name of company: Tabulated Trial Protocol Boehringer Ingelheim Name of finished product: Pradaxa® Name of active ingredient: Dabigatran Protocol date: Trial number: Revision date: 26 March 2014 1160.186 21 July 2016 Title of trial: A prospective Randomised, open label, blinded endpoint (PROBE) study to Evaluate DUAL antithrombotic therapy with dabigatran etexilate (110mg and 150mg b.i.d.) plus clopidogrel or ticagrelor vs. -

Emergency Management of Patients on Direct Oral Anticoagulants (Doacs)
Emergency Management of Patients on Direct Oral Anticoagulants (DOACs) Dr Tina Biss Consultant Haematologist Newcastle upon Tyne Hospitals NHS Foundation Trust NE RTC Annual Education Symposium 11 th October 2016 The extent of the problem ≈1-2% of the UK population are anticoagulated 70000 60000 AF 70% VTE 25% 50000 Other 5% 40000 30000 20000 10000 0 1996 1998 2000 2002 2004 2006 2008 2010 2012 2014 90 80 8% of individuals 70 >80 years of age are anticoagulated 60 50 40 30 20 10 0 0 20 40 60 80 100 Age distribution of patients on warfarin 2010 2016 Apixaban Rivaroxaban Warfarin Dabigatran Edoxaban Targets of Direct Oral Anticoagulants ORAL PARENTERAL TF/VIIa TTP889 TFPI (tifacogin) X IX Xa Inhibitors : IXa APC (drotrecogin alfa) VIIIa Rivaroxaban sTM (ART-123) Apixaban Edoxaban Va AT LY517717 Indirect Xa inhibitors YM150 Xa Fondaparinux PRT-054021 Idraparinux II SSR-126517 Direct Xa Inhibitors IIa Inhibitors DX-9065a Ximelagatran IIa Otamixaban Dabigatran Fibrinogen Fibrin TF=tissue factor Adapted from Weitz JI et al. J Thromb Haemost. 2005;3:1843-1853. The ideal anticoagulants? • Oral administration • Rapid onset of action • Relatively short half-life • Predictable pharmacokinetics No need for monitoring • Few drug or dietary interactions • Modest risk of bleeding • Rapidly reversible Predictable dose-response relationship No monitoring required Few drug interactions No dietary interactions Current agents and licensed indications Dabigatran Rivaroxaban Apixaban Edoxaban (IIa inhibitor (Xa inhibitor) (Xa inhibitor) (Xa inhibitor) -

202439Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202439Orig1s000 MEDICAL REVIEW(S) Clinical Review: Nhi Beasley, Preston Dunnmon and Martin Rose Application type: Standard, NDA 22-439 Xarelto (rivaroxaban) CLINICAL REVIEW Application Type NDA Type 1 -- (505(b)(1)) Application Number(s) 202439 Priority or Standard Standard Submit Date(s) 4 January 2011 Received Date(s) 5 January 2011 PDUFA Goal Date 5 November 2011 Division / Office DCRP/ODE 1 Reviewer Name(s) Nhi Beasley, Preston Dunnmon (safety); Martin Rose (efficacy) Review Completion Date 10 August 2011 Established Name Rivaroxaban Trade Name Xarelto® Therapeutic Class Anticoagulant (factor Xa inhibitor) Applicant Johnson & Johnson Pharmaceutical Research & Development, LLC Formulation(s) Oral tablets – 15 & 20 mg Dosing Regimen 15 or 20 mg once daily, based on renal function Indication(s) Prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation Intended Population(s) Adults Template Version: March 6, 2009 1 Reference ID: 2998874 Clinical Review: Nhi Beasley, Preston Dunnmon and Martin Rose Application type: Standard, NDA 22-439 Xarelto (rivaroxaban) Note to Readers In this review, a high level summary of the efficacy, safety and risk-benefit data is found in Section 1.2 Individual summaries of the efficacy and safety data are found at the beginning of Section 6 and Section 7, respectively. Internal hyperlinks to other parts of the review are in blue font. The Tables of Contents, Tables, and Figures are also hyperlinked to their targets. Table of Contents NOTE TO READERS ...................................................................................................... 2 TABLE OF CONTENTS .................................................................................................. 2 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ....................................... 10 1.1 Recommendation on Regulatory Action .......................................................... -

Horizons in Novel Oral Anticoagulation Therapy In
maco har log P y: r O la u p c e n s a A v Shihadeh et al., Cardiol Pharmacol 2015, 4:4 c o c i e d r s a s Open Access C Cardiovascular Pharmacology: DOI: 10.4172/2329-6607.1000155 ISSN: 2329-6607 Review Article OpenOpen Access Access Horizons in Novel Oral Anticoagulation Therapy in Concomitant Acute Coronary Syndromes and Atrial Fibrillation Leydimar Anmad Shihadeh, Diego Fernández-Rodríguez*, Javier Lorenzo-González and Julio Hernández-Afonso Cardiology Department, Nuestra Señora de Candelaria University Hospital, Santa Cruz de Tenerife, Spain Abstract Thrombus formation and coronary artery occlusion, in acute coronary syndromes, occur as a result of an atherosclerotic plaque rupture/erosion and the subsequent activation of platelets and coagulation factors. Also, cardioembolic events, in atrial fibrillation, are related to the thrombus formation and the systemic arterial embolization secondary to the blood stasis in left atrium. Antiplatelet treatments in acute coronary syndromes and long-term oral anticoagulation in atrial fibrillation have improved prognosis by reducing ischemic events but both treatments are associated with an increase in the risk of bleeding. Furthermore, thrombin and activated factor X are the key elements in the coagulation cascade and novel oral anticoagulants act by inhibiting these coagulation factors, generating a double effect: the reduction of ischemic events and the increment in hemorrhagic events. To date, the clinical benefit of novel oral anticoagulants, in patients presenting acute coronary syndromes and atrial fibrillation, has not well studied. For that reason, the objective of this manuscript is to explain basic clinical trials testing novel oral anticoagulants in patients with acute coronary syndromes and ongoing trials evaluating the use of new oral anticoagulants in population with acute coronary syndromes and atrial fibrillation: the PIONEER AF-PCI (Rivaroxaban), the RT-AF (Rivaroxaban) and the REDUAL-PCI (Dabigatran) trials. -

Study Protocol
Protocol 3-001 Confidential 28APRIL2017 Version 4.1 Asahi Kasei Pharma America Corporation Synopsis Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Assess the Safety and Efficacy of ART-123 in Subjects with Severe Sepsis and Coagulopathy Name of Sponsor/Company: Asahi Kasei Pharma America Corporation Name of Investigational Product: ART-123 Name of Active Ingredient: thrombomodulin alpha Objectives Primary: x To evaluate whether ART-123, when administered to subjects with bacterial infection complicated by at least one organ dysfunction and coagulopathy, can reduce mortality. x To evaluate the safety of ART-123 in this population. Secondary: x Assessment of the efficacy of ART-123 in resolution of organ dysfunction in this population. x Assessment of anti-drug antibody development in subjects with coagulopathy due to bacterial infection treated with ART-123. Study Center(s): Phase of Development: Global study, up to 350 study centers Phase 3 Study Period: Estimated time of first subject enrollment: 3Q 2012 Estimated time of last subject enrollment: 3Q 2018 Number of Subjects (planned): Approximately 800 randomized subjects. Page 2 of 116 Protocol 3-001 Confidential 28APRIL2017 Version 4.1 Asahi Kasei Pharma America Corporation Diagnosis and Main Criteria for Inclusion of Study Subjects: This study targets critically ill subjects with severe sepsis requiring the level of care that is normally associated with treatment in an intensive care unit (ICU) setting. The inclusion criteria for organ dysfunction and coagulopathy must be met within a 24 hour period. 1. Subjects must be receiving treatment in an ICU or in an acute care setting (e.g., Emergency Room, Recovery Room).