The Marked Reduction of the Indus River Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INDUS DELTA, PAKISTAN: Economic Costs of Reduction in Freshwater Flows
water allocationdecisions. factored intoriverbasinplanning,or benefits of water-basedecosystemsarerarely economic users ofwater.Yettheeconomic schemes, Pakistan’secosystems,too,are hydropower dams, reservoirs,irrigationand as water tolarge-scale,commercialusessuch imperative that favours theallocationof Contrary tothedominantdevelopment economically norecologicallyoptimal. decisions beingmadethatareneither needs has oftenledtowaterallocation Failure torecognisedownstreamecosystem heavily byupstreamwaterabstraction. end of rivers,havebeenimpactedmost the at lie and marineregions,becausethey Coastal ecosystems. needs ofdownstream many cases, left insufficientflowtomeetthe of large volumesofwaterfromrivershas,in particular there isconcernthattheabstraction exacting a heavytollontheenvironment.In This impressive irrigationsystemis,however, world. the irrigated torain-fedlandratioin highest the farmland, affordingPakistan system feedsmorethan15millionhectaresof than 1.65 million km(IRIN2001).The more watercourses witharunninglengthof 89,000 conveyance lengthof57,000km,and head works, 43maincanalswitha or barrages 19 three majorstoragereservoirs, comprises Pakistan’s vastirrigationnetwork Pakistan Water-based developmentsin flows reduction infreshwater economic costsof INDUS DELTA,PAKISTAN: VALUATION #5:May2003 CASE STUDIESINWETLAND Integrating Wetland Economic Values into River Basin Management Managing freshwater flows in the The economic costs and losses arising from Indus River such omissions can be immense, and often The Indus River has -

PESA-DP-Hyderabad-Sindh.Pdf
Rani Bagh, Hyderabad “Disaster risk reduction has been a part of USAID’s work for decades. ……..we strive to do so in ways that better assess the threat of hazards, reduce losses, and ultimately protect and save more people during the next disaster.” Kasey Channell, Acting Director of the Disaster Response and Mitigation Division of USAID’s Office of U.S. Foreign Disas ter Ass istance (OFDA) PAKISTAN EMERGENCY SITUATIONAL ANALYSIS District Hyderabad August 2014 “Disasters can be seen as often as predictable events, requiring forward planning which is integrated in to broader de velopment programs.” Helen Clark, UNDP Administrator, Bureau of Crisis Preven on and Recovery. Annual Report 2011 Disclaimer iMMAP Pakistan is pleased to publish this district profile. The purpose of this profile is to promote public awareness, welfare, and safety while providing community and other related stakeholders, access to vital information for enhancing their disaster mitigation and response efforts. While iMMAP team has tried its best to provide proper source of information and ensure consistency in analyses within the given time limits; iMMAP shall not be held responsible for any inaccuracies that may be encountered. In any situation where the Official Public Records differs from the information provided in this district profile, the Official Public Records should take as precedence. iMMAP disclaims any responsibility and makes no representations or warranties as to the quality, accuracy, content, or completeness of any information contained in this report. Final assessment of accuracy and reliability of information is the responsibility of the user. iMMAP shall not be liable for damages of any nature whatsoever resulting from the use or misuse of information contained in this report. -

The Geographic, Geological and Oceanographic Setting of the Indus River
16 The Geographic, Geological and Oceanographic Setting of the Indus River Asif Inam1, Peter D. Clift2, Liviu Giosan3, Ali Rashid Tabrez1, Muhammad Tahir4, Muhammad Moazam Rabbani1 and Muhammad Danish1 1National Institute of Oceanography, ST. 47 Clifton Block 1, Karachi, Pakistan 2School of Geosciences, University of Aberdeen, Aberdeen AB24 3UE, UK 3Geology and Geophysics, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA 4Fugro Geodetic Limited, 28-B, KDA Scheme #1, Karachi 75350, Pakistan 16.1 INTRODUCTION glaciers (Tarar, 1982). The Indus, Jhelum and Chenab Rivers are the major sources of water for the Indus Basin The 3000 km long Indus is one of the world’s larger rivers Irrigation System (IBIS). that has exerted a long lasting fascination on scholars Seasonal and annual river fl ows both are highly variable since Alexander the Great’s expedition in the region in (Ahmad, 1993; Asianics, 2000). Annual peak fl ow occurs 325 BC. The discovery of an early advanced civilization between June and late September, during the southwest in the Indus Valley (Meadows and Meadows, 1999 and monsoon. The high fl ows of the summer monsoon are references therein) further increased this interest in the augmented by snowmelt in the north that also conveys a history of the river. Its source lies in Tibet, close to sacred large volume of sediment from the mountains. Mount Kailas and part of its upper course runs through The 970 000 km2 drainage basin of the Indus ranks the India, but its channel and drainage basin are mostly in twelfth largest in the world. Its 30 000 km2 delta ranks Pakiistan. -
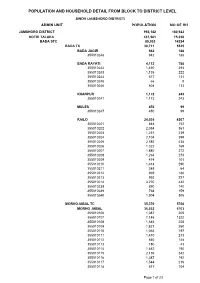
Jamshoro Blockwise
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL SINDH (JAMSHORO DISTRICT) ADMIN UNIT POPULATION NO OF HH JAMSHORO DISTRICT 993,142 180,922 KOTRI TALUKA 437,561 75,038 BADA STC 85,033 14234 BADA TC 30,711 5525 BADA JAGIR 942 188 355010248 942 188 BADA RAYATI 4,112 788 355010242 1,430 294 355010243 1,135 222 355010244 677 131 355010245 66 8 355010246 804 133 KHANPUR 1,173 243 355010241 1,173 243 MULES 450 99 355010247 450 99 RAILO 24,034 4207 355010201 844 152 355010202 2,054 361 355010203 1,251 239 355010204 2,104 399 355010205 2,585 438 355010206 1,022 169 355010207 1,880 272 355010208 1,264 273 355010209 474 101 355010210 1,414 290 355010211 348 64 355010212 969 186 355010213 993 227 355010214 3,270 432 355010238 890 140 355010239 768 159 355010240 1,904 305 MORHOJABAL TC 35,370 5768 MORHO JABAL 35,032 5703 355010106 1,087 205 355010107 7,146 1322 355010108 1,646 228 355010109 1,821 260 355010110 1,065 197 355010111 1,410 213 355010112 840 148 355010113 180 43 355010114 1,462 190 355010115 2,136 342 355010116 1,387 192 355010117 1,544 216 355010118 617 104 Page 1 of 23 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL SINDH (JAMSHORO DISTRICT) ADMIN UNIT POPULATION NO OF HH 355010119 79 15 355010120 3,665 538 355010121 951 129 355010122 2,161 343 355010123 2,169 355 355010124 1,468 261 355010125 2,198 402 TARBAND 338 65 355010126 338 65 PETARO TC 18,952 2941 ANDHEJI-KASI 1,541 292 355010306 1,541 292 BELO GHUGH 665 134 355010311 665 134 MANJHO JAGIR 659 123 355010307 659 123 MANJHO RAYATI 1,619 306 355010310 1,619 -

Himalaya to the Sea: Geology, Geomorphology and the Quaternary' by John F
HIMALAYA, the Journal of the Association for Nepal and Himalayan Studies Volume 16 Number 1 Himalayan Research Bulletin No. 1 & Article 17 2 1996 Book review of 'Himalaya to the Sea: Geology, Geomorphology and the Quaternary' by John F. Shroder, Jr. (ed.) Daniel D. Schelling University of Utah Follow this and additional works at: https://digitalcommons.macalester.edu/himalaya Recommended Citation Schelling, Daniel D.. 1996. Book review of 'Himalaya to the Sea: Geology, Geomorphology and the Quaternary' by John F. Shroder, Jr. (ed.). HIMALAYA 16(1). Available at: https://digitalcommons.macalester.edu/himalaya/vol16/iss1/17 This Book Review is brought to you for free and open access by the DigitalCommons@Macalester College at DigitalCommons@Macalester College. It has been accepted for inclusion in HIMALAYA, the Journal of the Association for Nepal and Himalayan Studies by an authorized administrator of DigitalCommons@Macalester College. For more information, please contact [email protected]. William Fisher Harvard University Himalaya to the Sea: Geology, Geomorphology and the Quaternary. Edited by John F. Shroder, Jr. London, Routledge, 1993. Pp. xxvii + 429. $130.00 Located along the northwestern sector of the within the Himalayan region and South Asia (on which Himalayan continental-collision belt, the Kirthar many volumes have been published in recent years), and Sulaiman transform plate-boundary, and the eastern therefore the collection of papers in this volume will be sector of the Makran oceanic-subduction zone, Pakistan of interest to earth scientists working in a large number is one of the most tectonically active regions in the of tectonic, sedimentological and geomorphological world. -

“Major World Deltas: a Perspective from Space
“MAJOR WORLD DELTAS: A PERSPECTIVE FROM SPACE” James M. Coleman Oscar K. Huh Coastal Studies Institute Louisiana State University Baton Rouge, LA TABLE OF CONTENTS Page INTRODUCTION……………………………………………………………………4 Major River Systems and their Subsystem Components……………………..4 Drainage Basin………………………………………………………..7 Alluvial Valley………………………………………………………15 Receiving Basin……………………………………………………..15 Delta Plain…………………………………………………………...22 Deltaic Process-Form Variability: A Brief Summary……………………….29 The Drainage Basin and The Discharge Regime…………………....29 Nearshore Marine Energy Climate And Discharge Effectiveness…..29 River-Mouth Process-Form Variability……………………………..36 DELTA DESCRIPTIONS…………………………………………………………..37 Amu Darya River System………………………………………………...…45 Baram River System………………………………………………………...49 Burdekin River System……………………………………………………...53 Chao Phraya River System……………………………………….…………57 Colville River System………………………………………………….……62 Danube River System…………………………………………………….…66 Dneiper River System………………………………………………….……74 Ebro River System……………………………………………………..……77 Fly River System………………………………………………………...…..79 Ganges-Brahmaputra River System…………………………………………83 Girjalva River System…………………………………………………….…91 Krishna-Godavari River System…………………………………………… 94 Huang He River System………………………………………………..……99 Indus River System…………………………………………………………105 Irrawaddy River System……………………………………………………113 Klang River System……………………………………………………...…117 Lena River System……………………………………………………….…121 MacKenzie River System………………………………………………..…126 Magdelena River System……………………………………………..….…130 -

Budget Execution Report 2Nd QUARTER 2020-21
Budget Execution Report 2nd QUARTER 2020-21 31th December, 2020 Government of Sindh Finance Department Table of contents: Introduction ............................................................................................................................................................................. 2 Table 1 Interim Fiscal Statement .......................................................................................................................................... 3 Table 2 Revenue by Object .................................................................................................................................................... 4 Table 3 Revenue by Department........................................................................................................................................... 7 Table 4 Expenditure by Department .................................................................................................................................... 9 Table 5 Recurrent Expenditure by Department, Grant and Object ............................................................................... 20 Table 6 Provincial ADP by Sector and Sub-sector .......................................................................................................... 41 Table 7 Development Expenditure by Sector, Subsector and Scheme ....................................................................... 42 Table 8 Current Capital Expenditure ............................................................................................................................... -

Table of Contents
TABLE OF CONTENTS 1. Introductionp. 1 2. Political developments since early 1992p. 1 3. Amnesty International's work on torture, deaths in custody, extrajudicial executions and "disappearances" in Pakistan since 1991p. 3 4. Methods of torture employed in Pakistanp. 4 4.1 Rape in custodyp.10 5. Deaths in custody, extrajudicial executions and "disappearances"p.13 6. Reasons for the use of torture in Pakistanp.19 7. The prohibition of arbitrary arrest, torture, extrajudicial executions and "disappearances" in Pakistan's national law and in international lawp.20 8. Amnesty International's recommendations regarding safeguards against torture, extrajudicial killings and "disappearances" in Pakistanp.23 Appendix A: Cases of torture, death in custody and extrajudicial execution in Pakistan in 1992 and 1993p.29 1. Illegal detention and torture of Ghulam Mustafa Soomro p.29 2. Illegal detention and torture of Inderjit Lohanap.31 3. Death of Bebal Khatoon Shirazip.32 4. Death of Nazir Masih p.33 5. Illegal detention and torture of labourers and their families in rural private jailsp.34 6. A political party, the Mohajir Qaumi Movement (MQM), as perpetrator and victim of human rights violationsp.37 7. Illegal detention, torture and extrajudicial execution of Niaz Hussain Amnesty International December 1993AI Index: ASA 33/05/93 Pakistan: Torture and deaths in custody Pathan p.43 8. Illegal detention, torture and death in custody or extrajudicial execution of Mujib Aijaz Jatoip.46 9. Extrajudicial executions of nine men at Tando Bahawalp.47 10. Reported torture and extrajudicial executions of seven young men at Shah Bandarp.49 11. Illegal detention, torture and death in custody or extrajudicial execution of Yusuf Jakhrani p.52 12. -

Three Decades of Coastal Changes in Sindh, Pakistan (1989–2018): a Geospatial Assessment
remote sensing Article Three Decades of Coastal Changes in Sindh, Pakistan (1989–2018): A Geospatial Assessment Shamsa Kanwal 1,* , Xiaoli Ding 1 , Muhammad Sajjad 2,3 and Sawaid Abbas 1 1 Department of Land Surveying and Geo-Informatics, The Hong Kong Polytechnic University, Hong Kong, China; [email protected] (X.D.); [email protected] (S.A.) 2 Guy Carpenter Asia-Pacific Climate Impact Centre, School of Energy and Environment, City University of Hong Kong, Hong Kong, China; [email protected] 3 Department of Civil and Environmental Engineering, Princeton University, Princeton, NJ 08542, USA * Correspondence: [email protected] Received: 3 November 2019; Accepted: 13 December 2019; Published: 18 December 2019 Abstract: Coastal erosion endangers millions living near-shore and puts coastal infrastructure at risk, particularly in low-lying deltaic coasts of developing nations. This study focuses on morphological changes along the ~320-km-long Sindh coastline of Pakistan over past three decades. In this study, the Landsat images from 1989 to 2018 at an interval of 10 years are used to analyze the state of coastline erosion. For this purpose, well-known statistical approaches such as end point rate (EPR), least median of squares (LMS), and linear regression rate (LRR) are used to calculate the rates of coastline change. We analyze the erosion trend along with the underlying controlling variables of coastal change. Results show that most areas along the coastline have experienced noteworthy erosion during the study period. It is found that Karachi coastline experienced 2.43 0.45 m/yr of erosion and ± 8.34 0.45 m/yr of accretion, while erosion on the western and eastern sides of Indus River reached ± 12.5 0.55 and 19.96 0.65 m/yr on average, respectively. -

Climate Change Profile of Pakistan
Climate Change Profi le of Pakistan Catastrophic fl oods, droughts, and cyclones have plagued Pakistan in recent years. The fl ood killed , people and caused around billion in damage. The Karachi heat wave led to the death of more than , people. Climate change-related natural hazards may increase in frequency and severity in the coming decades. Climatic changes are expected to have wide-ranging impacts on Pakistan, a ecting agricultural productivity, water availability, and increased frequency of extreme climatic events. Addressing these risks requires climate change to be mainstreamed into national strategy and policy. This publication provides a comprehensive overview of climate change science and policy in Pakistan. About the Asian Development Bank ADB’s vision is an Asia and Pacifi c region free of poverty. Its mission is to help its developing member countries reduce poverty and improve the quality of life of their people. Despite the region’s many successes, it remains home to a large share of the world’s poor. ADB is committed to reducing poverty through inclusive economic growth, environmentally sustainable growth, and regional integration. Based in Manila, ADB is owned by members, including from the region. Its main instruments for helping its developing member countries are policy dialogue, loans, equity investments, guarantees, grants, and technical assistance. CLIMATE CHANGE PROFILE OF PAKISTAN ASIAN DEVELOPMENT BANK 6 ADB Avenue, Mandaluyong City 1550 Metro Manila, Philippines ASIAN DEVELOPMENT BANK www.adb.org Prepared by: Qamar Uz Zaman Chaudhry, International Climate Technology Expert ASIAN DEVELOPMENT BANK Creative Commons Attribution 3.0 IGO license (CC BY 3.0 IGO) © 2017 Asian Development Bank 6 ADB Avenue, Mandaluyong City, 1550 Metro Manila, Philippines Tel +63 2 632 4444; Fax +63 2 636 2444 www.adb.org Some rights reserved. -

Table of Contents
Environmental and Social Management Framework (ESMF) Draft Pakistan Hydro-Meteorological and DRM Services Project Pakistan Meteorological Department National Disaster Management Authority Pakistan Hydro-Meteorological and DRM Services Project Executive Summary Background Climate change is expected to have an adverse impact on Pakistan, as it ranks 7th on the climate risk index. It continues to be one of the most flood-prone countries in the South Asia Region (SAR); suffering US$18 billion in losses between 2005 and 2014 (US$10.5 billion from the 2010 floods alone), equivalent to around 6% of the federal budget. Hydromet hazards have been coupled with rapid population growth and uncontrolled urbanization, leading to a disproportionate and growing impact on the poor. To build on recent development gains, increase economic productivity, and improve climate resilience, it will be critical to improve the quality and accessibility of weather, water, and climate information services. Climate-resilient development requires stronger institutions and a higher level of observation, forecasting, and service delivery capacity; these could make a significant contribution to safety, security, and economic well-being. The Pakistan Hydro- Meteorological and DRM Services Project (PHDSP) expects to improve hydro- meteorological information and services, strengthen forecasting and early warning systems, and improve dissemination of meteorological and hydrological forecasts, warnings and advisory information to stakeholders and end-users and strengthen the existing disaster risk management (DRM) capacity and services of the National Disaster Management Authority (NDMA). Project Description The project has three main components and will be implemented over a period of five years. Component 1: Hydro-Meteorological and Climate Services The objective of this component is to improve the capability and thereby performance of the PMD to understand and make use of meteorological and hydrological information for decision making. -

Effect of River Indus Flow on Low Riparian Ecosystems of Sindh: a Review Paper
Rec. Zool. Surv. Pakistan 21: 86-89 (2012) Effect of River Indus flow on low riparian ecosystems of Sindh: a review paper Syed Mehmood Nasir1, Ghulam Akbar2 1Ministry of Climate Change, 5th floor, LG & RD Complex, G-5/2, Islamabad, Pakistan 2WWF - Pakistan, House No. 451, Street No. 2, Sector F-11/1, Islamabad, Pakistan Corresponding author: Syed Mehmood Nasir (Email: [email protected]) KEYWORDS ABSTRACT Indus River flow The present study is focused on the threats to low riparian ecosystems of Indus River emanating from Indus delta deteriorating river flow regime coupled with associated anthropogenic activities. Indus River which serves as the lifeline of freshwater in the country is not only significant for the agricultural production and drinking Indus Basin water for the human, animals and plant survival but also for the perpetuity of riverine and deltaic ecology. Low riparian ecosystem The water flow pattern of the Indus River has been constantly changing with the changing climatic conditions Indus eco-region and the human activities especially in the upstream areas. Substantial water diversion in the upper riparian Sustainable strategies zone has resulted in serious threats to the low riparian ecosystems and the sustenance of the associated local communities. This has led to the problems of over-exploitation of the ground water resources and Riverine areas degradation of quality of water. Formulating and ensuring effective implementation of sustainable strategies Coastal areas for the management of Indus River flow can only solve these issues. Introduction Unfortunately, the upstream diversions of River Indus by unchecked development of water infrastructure during 20th Water being a vital component for life is facing serious issues in century have caused gradual reduction in the water flow of river.