An Overview for the Layperson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
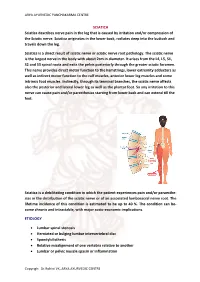
SCIATICA Sciatica Describes Nerve Pain in the Leg That Is Caused by Irritation And/Or Compression of the Sciatic Nerve
ARYA AYURVEDIC PANCHAKARMA CENTRE SCIATICA Sciatica describes nerve pain in the leg that is caused by irritation and/or compression of the Sciatic nerve. Sciatica originates in the lower back, radiates deep into the buttock and travels down the leg. Sciatica is a direct result of sciatic nerve or sciatic nerve root pathology. The sciatic nerve is the largest nerve in the body with about 2cm in diameter. It arises from the L4, L5, S1, S2 and S3 spinal roots and exits the pelvis posteriorly through the greater sciatic foramen. This nerve provides direct motor function to the hamstrings, lower extremity adductors as well as indirect motor function to the calf muscles, anterior lower leg muscles and some intrinsic foot muscles. Indirectly, through its terminal branches, the sciatic nerve affects also the posterior and lateral lower leg as well as the plantar foot. So any irritation to this nerve can cause pain and/or paresthesias starting from lower back and can extend till the feet. Sciatica is a debilitating condition in which the patient experiences pain and/or paraesthe- sias in the distribution of the sciatic nerve or of an associated lumbosacral nerve root. The lifetime incidence of this condition is estimated to be up to 40 %. The condition can be- come chronic and intractable, with major socio-economic implications. ETIOLOGY • Lumbar spinal stenosis • Herniated or bulging lumbar intervertebral disc • Spondylolisthesis • Relative misalignment of one vertebra relative to another • Lumbar or pelvic muscle spasm or inflammation Copyrigh: Dr.Rohini VK, ARYA AYURVEDIC CENTRE ARYA AYURVEDIC PANCHAKARMA CENTRE • Spinal or Paraspinal masses included malignancy, epidural haematoma or epidural abscess • Lumbar degenerative disc disease • Sacroiliac joint dysfunction EPIDEMIOLOGY GENDER: There appears to be no gender predominance. -

(Kir) Channels in Tick Salivary Gland Function Zhilin Li Louisiana State University and Agricultural and Mechanical College, [email protected]
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 3-26-2018 Characterizing the Physiological Role of Inward Rectifier Potassium (Kir) Channels in Tick Salivary Gland Function Zhilin Li Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Entomology Commons Recommended Citation Li, Zhilin, "Characterizing the Physiological Role of Inward Rectifier Potassium (Kir) Channels in Tick Salivary Gland Function" (2018). LSU Master's Theses. 4638. https://digitalcommons.lsu.edu/gradschool_theses/4638 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. CHARACTERIZING THE PHYSIOLOGICAL ROLE OF INWARD RECTIFIER POTASSIUM (KIR) CHANNELS IN TICK SALIVARY GLAND FUNCTION A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Entomology by Zhilin Li B.S., Northwest A&F University, 2014 May 2018 Acknowledgements I would like to thank my family (Mom, Dad, Jialu and Runmo) for their support to my decision, so I can come to LSU and study for my degree. I would also thank Dr. Daniel Swale for offering me this awesome opportunity to step into toxicology filed, ask scientific questions and do fantastic research. I sincerely appreciate all the support and friendship from Dr. -

Radiculopathy Vs. Spinal Stenosis: Evocative Electrodiagnosis Identifies the Main Pain Generator
Functional Electromyography Loren M. Fishman · Allen N. Wilkins Functional Electromyography Provocative Maneuvers in Electrodiagnosis 123 Loren M. Fishman, MD Allen N. Wilkins, MD College of Physicians & Surgeons Manhattan Physical Medicine Columbia University and Rehabilitation New York, NY 10028, USA New York, NY 10013, USA [email protected] ISBN 978-1-60761-019-9 e-ISBN 978-1-60761-020-5 DOI 10.1007/978-1-60761-020-5 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010935087 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. -

Piriformis Syndrome: the Literal “Pain in My Butt” Chelsea Smith, PTA
Piriformis Syndrome: the literal “pain in my butt” Chelsea Smith, PTA Aside from the monotony of day-to-day pains and annoyances, piriformis syndrome is the literal “pain in my butt” that may not go away with sending the kids to grandmas and often takes the form of sciatica. Many individuals with pain in the buttock that radiates down the leg are experiencing a form of sciatica caused by irritation of the spinal nerves in or near the lumbar spine (1). Other times though, the nerve irritation is not in the spine but further down the leg due to a pesky muscle called the piriformis, hence “piriformis syndrome”. The piriformis muscle is a flat, pyramidal-shaped muscle that originates from the front surface of the sacrum and the joint capsule of the sacroiliac joint (SI joint) and is located deep in the gluteal tissue (2). The piriformis travels through the greater sciatic foramen and attaches to the upper surface of the greater trochanter (or top of the hip bone) while the sciatic nerve runs under (and sometimes through) the piriformis muscle as it exits the pelvis. Due to this close proximity between the piriformis muscle and the sciatic nerve, if there is excessive tension (tightness), spasm, or inflammation of the piriformis muscle this can cause irritation to the sciatic nerve leading to symptoms of sciatica (pain down the leg) (1). Activities like sitting on hard surfaces, crouching down, walking or running for long distances, and climbing stairs can all increase symptoms (2) with the most common symptom being tenderness along the piriformis muscle (deep in the gluteal region) upon palpation. -

Neuropathy, Radiculopathy & Myelopathy
Neuropathy, Radiculopathy & Myelopathy Jean D. Francois, MD Neurology & Neurophysiology Purpose and Objectives PURPOSE Avoid Confusing Certain Key Neurologic Concepts OBJECTIVES • Objective 1: Define & Identify certain types of Neuropathies • Objective 2: Define & Identify Radiculopathy & its causes • Objective 3: Define & Identify Myelopathy FINANCIAL NONE DISCLOSURE Basics What is Neuropathy? • The term 'neuropathy' is used to describe a problem with the nerves, usually the 'peripheral nerves' as opposed to the 'central nervous system' (the brain and spinal cord). It refers to Peripheral neuropathy • It covers a wide area and many nerves, but the problem it causes depends on the type of nerves that are affected: • Sensory nerves (the nerves that control sensation>skin) causing cause tingling, pain, numbness, or weakness in the feet and hands • Motor nerves (the nerves that allow power and movement>muscles) causing weakness in the feet and hands • Autonomic nerves (the nerves that control the systems of the body eg gut, bladder>internal organs) causing changes in the heart rate and blood pressure or sweating • It May produce Numbness, tingling,(loss of sensation) along with weakness. It can also cause pain. • It can affect a single nerve (mononeuropathy) or multiple nerves (polyneuropathy) Neuropathy • Symptoms usually start in the longest nerves in the body: Feet & later on the hands (“Stocking-glove” pattern) • Symptoms usually spread slowly and evenly up the legs and arms. Other body parts may also be affected. • Peripheral Neuropathy can affect people of any age. But mostly people over age 55 • CAUSES: Neuropathy has a variety of forms and causes. (an injury systemic illness, an infection, an inherited disorder) some of the causes are still unknown. -

F-MARC Football Medicine Manual 2Nd Edition F-MARC Football Medicine Manual 2Nd Edition 2 Editors - Authors - Contributors | Football Medicine Manual
F-MARC Football Medicine Manual 2nd Edition F-MARC Football Medicine Manual 2nd Edition 2 Editors - Authors - Contributors | Football Medicine Manual Football Medicine Manual Editors DVORAK Jiri Prof. Dr F-MARC, Schulthess Clinic Zurich, Switzerland JUNGE Astrid Dr F-MARC, Schulthess Clinic Zurich, Switzerland GRIMM Katharina Dr FIFA Medical Offi ce Zurich, Switzerland Authors 2nd Edition 2009 ACKERMAN Kathryn E. Harvard Medical School Harvard, USA BABWAH Terence Dr Sports Medicine and Injury Rehabilitation Clinic Macoya, Trinidad BAHR Roald Prof. Dr Oslo Sports Trauma Research Center Oslo, Norway BANGSBO Jens Prof. Dr University of Copenhagen Copenhagen, Denmark BÄRTSCH Peter Prof. Dr University of Heidelberg Heidelberg, Germany BIZZINI Mario PT Schulthess Clinic Zurich, Switzerland CHOMIAK Jiri Dr Orthopaedic University Hospital Bulovka Prague, Czech Republic DVORAK Jiri Prof. Dr F-MARC, Schulthess Klinik Zurich, Switzerland EDWARDS Tony Dr Adidas Sports Medicine Auckland, New Zealand ENGEBRETSEN Lars Prof. Dr Oslo Sports Trauma Research Center Oslo, Norway FULLER Colin Prof. Dr University of Nottingham Nottingham, England GRIMM Katharina Dr FIFA Medical Offi ce Zurich, Switzerland JUNGE Astrid Dr F-MARC, Schulthess Clinic Zurich, Switzerland KHAN Karim Prof. Dr Editor in Chief British Journal of Sports Medicine Sydney, Australia Editors - Authors - Contributors | Football Medicine Manual 3 KOLBE John Prof. Dr University of Auckland Auckland, New Zealand LÜSCHER Thomas Prof. Dr University of Zurich Zurich, Switzerland MANDELBAUM Bert Dr Santa Monica Orthopaedic and Sports Medicine Group Santa Monica, USA MAUGHAN Ron Prof. Dr University of Loughborough Loughborough, Great Britain PETERSON Lars Prof. Dr Gothenburg Medical Center Gothenburg, Sweden REILLY Thomas Prof. Dr Liverpool John Moores University Liverpool, Great Britain SALTIN Bengt Prof. -

Hereditary Spastic Paraparesis: a Review of New Developments
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.69.2.150 on 1 August 2000. Downloaded from 150 J Neurol Neurosurg Psychiatry 2000;69:150–160 REVIEW Hereditary spastic paraparesis: a review of new developments CJ McDermott, K White, K Bushby, PJ Shaw Hereditary spastic paraparesis (HSP) or the reditary spastic paraparesis will no doubt Strümpell-Lorrain syndrome is the name given provide a more useful and relevant classifi- to a heterogeneous group of inherited disorders cation. in which the main clinical feature is progressive lower limb spasticity. Before the advent of Epidemiology molecular genetic studies into these disorders, The prevalence of HSP varies in diVerent several classifications had been proposed, studies. Such variation is probably due to a based on the mode of inheritance, the age of combination of diVering diagnostic criteria, onset of symptoms, and the presence or other- variable epidemiological methodology, and wise of additional clinical features. Families geographical factors. Some studies in which with autosomal dominant, autosomal recessive, similar criteria and methods were employed and X-linked inheritance have been described. found the prevalance of HSP/100 000 to be 2.7 in Molise Italy, 4.3 in Valle d’Aosta Italy, and 10–12 Historical aspects 2.0 in Portugal. These studies employed the In 1880 Strümpell published what is consid- diagnostic criteria suggested by Harding and ered to be the first clear description of HSP.He utilised all health institutions and various reported a family in which two brothers were health care professionals in ascertaining cases aVected by spastic paraplegia. The father was from the specific region. -

Toxicological Testing in Large Animals
Toxicological Testing in Large Animals Toxic causes of ill health and death in production animals are numerous. Toxin testing requires a specific toxin to be nominated as there is no suite of tests that covers all possibilities. Toxin testing is inherently expensive, requires specific sample types and false negatives can occur; for instance the toxin may have been eliminated from the body or be undetectable, but clinical signs may persist. Gribbles Veterinary Pathology can offer specific testing for a range of toxic substances, however it is important to consider the specific sample requirements and testing limitations for each toxin when advising your clients. Many tests are referred to external laboratories and may have extended turnaround times. Please contact the laboratory if you need testing for a specific toxin not listed here; we can often source unusual tests as needed from our network of referral laboratories. Clinicians should also consider syndromes which may mimic intoxication such as hypocalcaemia, hypoglycaemia, hepatic encephalopathy, peripheral neuropathies and primary CNS diseases. Examples of intoxicants that can be tested are provided below. See individual tests in the Pricelist for sample requirements and costs. Biological control agents Heavy metals • 1080 (fluoroacetate) • Arsenic • Strychnine • Lead • Synthetic pyrethroids • Copper • Organophosphates • Selenium • Organochlorines • Zinc • Carbamates • Metaldehyde • Anticoagulant rodenticides (warfarin, pindone, coumetetryl, bromadiolone, difenacoum, brodifacoum) -

GBS/CIDP Foundation International
Guillain-Barré Syndrome GBS: An Acute Care Guide For Medical Professionals A publication of the GBS/CIDP Foundation International Guillain-Barré Syndrome: An Acute Care Guide For Medical Professionals A publication of the GBS/CIDP Foundation International 2012 Edition GBS/CIDP Foundation International The Holly Building 104 1/2 Forrest Avenue Narberth, PA 19072 Phone: 610.667.0131 Toll Free: 866.224.3301 Fax: 610.667.7036 [email protected] www.gbs-cidp.org Guillain-Barré Syndrome: An Acute Care Guide For Medical Professionals Contents Page Acknowledgements . i Introduction . 1 Initial Patient Evaluation . 4 Natural History of GBS: Implications for Patient Care . 6 Respiratory Complications . 8 Dysautonomia and Cardiovascular Complications . 12 Bladder, Bowel Dysfunction . 14 Metabolism: Nutrition, Hydration, Electrolytes . 14 Pain . 17 ICU Delirium . 18 Skin . 18 Musculo-Skeletal Issues, Occupational and Physical Therapy . 19 Infection . 22 Disorder Specific Treatments . 22 Appendix A. Checklist of Patient Issues to Monitor . 24 B. Diagnostic Criteria for GBS . 25 C. Prognosis . 26 References . 27 This pamphlet is provided as a service of the GBS/CIDP Foundation International Serving the medical community and patients with Guillain-Barré syndrome and related acute and chronic paralyzing disorders of the peripheral nerves. Acknowledgements Guillain-Barré syndrome (GBS) is a rare disorder. Some health professionals may not be familiar with treating it. A beautiful video by Tanya Ooraikul chronicled the superb care provided to her husband Kit during his recovery from GBS. His care at Gray Nuns Community Hospital in Edmonton, Alberta, Canada included 86 days in the intensive care unit. The video handsomely demonstrates the high quality of care that can be provided for this rare and complicated disorder in a community hospital. -

Hereditary Spastic Paraplegia
8 Hereditary Spastic Paraplegia Notes and questions Hereditary Spastic Paraplegia What is Hereditary Spastic Paraplegia? Hereditary Spastic Paraplegia (HSP) is a medical term for a condition that affects muscle function. The terms spastic and paraplegia comes from several words in Greek: • ‘spastic’ means afflicted with spasms (an alteration in muscle tone that results in affected movements) • ‘paraplegia’ meaning an impairment in motor or sensory function of the lower extremities (from the hips down) What are the signs and symptoms of HSP? Muscular spasticity • Individuals with HSP commonly will have lower extremity weakness, spasticity, and muscle stiffness. • This can cause difficulty with walking or a “scissoring” gait. We are grateful to an anonymous donor for making a kind and Other common signs or symptoms include: generous donation to the Neuromuscular and Neurometabolic Centre. • urinary urgency • overactive or over responsive “brisk” reflexes © Hamilton Health Sciences, 2019 PD 9983 – 01/2019 Dpc/pted/HereditarySpasticParaplegia-trh.docx dt/January 15, 2019 ____________________________________________________________________________ 2 7 Hereditary Spastic Paraplegia Hereditary Spastic Paraplegia HSP is usually a chronic or life-long disease that affects If you have any questions about DM1, please speak with your people in different ways. doctor, genetic counsellor, or nurse at the Neuromuscular and Neurometabolic Centre. HSP can be classified as either “Uncomplicated HSP” or “Complicated HSP”. Notes and questions Types of Hereditary Spastic Paraplegia 1. Uncomplicated HSP: • Individuals often experience difficulty walking as the first symptom. • Onset of symptoms can begin at any age, from early childhood through late adulthood. • Symptoms may be non-progressive, or they may worsen slowly over many years. -
Unilateral Foot Drop: an Unusual Presentation of a More Common
DOI: 10.7860/JCDR/2017/26249.10738 Case Report Unilateral Foot Drop: An Unusual Section Presentation of a more Common Internal Medicine Disease RAMESHWAR NATH CHAURASIA1, ABHISHEK PathaK2, VIJAY nath MISHRA3, DEEPIKA JOSHI4 ABSTRACT An isolated and unilateral foot drop due to intracranial lesion is quite rare. Presenting herein a case of a 14-year-old female who complained of inability to wear and hold slipper in her left foot. Detailed neurological examination revealed left foot dorsiflexion which had 1/5 muscle power along with brisk left ankle reflex. Magnetic resonance imaging of the brain revealed multiple conglomerate inflammatory granulomas in cerebrum and cerebellum, larger one in right parasagittal region with perifocal oedema. Magnetic resonance spectrum was suggestive of tuberculoma. Her chest X-ray chest revealed milliary shadowing. She was put on anti- tubercular drugs, steroid and a prophylactic anti-epileptic drug. The dorsiflexion improved to grade 4/5 after three weeks of treatment. The motor homunculus for foot is located in parasagittal area. Therefore, in patients with foot drop, we must keep high index of suspicion for parasagittal lesions, so that prompt diagnosis and early management can be done to prevent complications and improve the quality of life of patient. Keywords: Lower motor neuron, Magnetic resonance imaging, Spastic foot drop, Tuberculoma, Upper motor neuron CASE REPORT A 14-year-old female presented with history of difficulty in walking for last three days after left foot drop. She noticed difficulty in her left foot when she was trying to wear shoes go to school. Weakness gradually progressed within next two days so much so that she was unable to hold slipper and clear the ground without tripping by her left foot. -

Dr Peter Heppner Consultant Neurosurgeon Auckland City Hospital Starship Childrens Hospital Ascot Hospital
Dr Peter Heppner Consultant Neurosurgeon Auckland City Hospital Starship Childrens Hospital Ascot Hospital 14:00 - 14:55 WS #55: Case Studies on Managing Cervical Radiculopathy 15:05 - 16:00 WS #67: Case Studies on Managing Cervical Radiculopathy (Repeated) Case Studies on Managing Cervical Radiculopathy: Peter Heppner Neurosurgeon Auckland City Hospital Starship Childrens Hospital Ascot Private Hospital www.neurosurgeon.org.nz DISCLOSURES I have no actual or potential conflict of interest in relation to this presentation WHAT ARE THE TAKE HOME POINTS? Evidence relating to cervical radiculopathy management is poor Natural history is generally very good In the absence of red flags, initial management with analgesia and physiotherapy appropriate NRIs can be a useful therapeutic and diagnostic tool Surgery ideally considered between 3-6 months from onset Either anterior or posterior surgical approaches can be selected depending on specifics of the case CASE 1 58 yr old lady 2 weeks radiating left arm pain (?after pilates) Taking paracetamol and NSAID Mild parasthesia in thumb Neuro exam normal Neck Disability Index 28% (mild) Clinically: Mild C6 radiculopathy of short duration CERVICAL RADICULOPATHY Radiating arm pain in a nerve distribution due to mechanical compression/chemical irritation of the nerve root Referred pain to inter-scapular and lateral neck common Weakness usually mild Pain or parasthesia non-dermatomal in almost half of patients Reduced reflex best predictor of imaging findings>motor weakness>sensory