A Selected Thermodynamic Database for REE to Be Used in HLNW Performance Assessment Exercises
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore
Minerals 2015, 5, 189-202; doi:10.3390/min5020189 OPEN ACCESS minerals ISSN 2075-163X www.mdpi.com/journal/minerals Article Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore Doyun Shin 1,2,*, Jiwoong Kim 1, Byung-su Kim 1,2, Jinki Jeong 1,2 and Jae-chun Lee 1,2 1 Mineral Resources Resource Division, Korea Institute of Geoscience and Mineral Resources (KIGAM), Gwahangno 124, Yuseong-gu, Daejeon 305-350, Korea; E-Mails: [email protected] (J.K.); [email protected] (B.K.); [email protected] (J.J.); [email protected] (J.L.) 2 Department of Resource Recycling Engineering, Korea University of Science and Technology, Gajeongno 217, Yuseong-gu, Daejeon 305-350, Korea * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +82-42-868-3616. Academic Editor: Anna H. Kaksonen Received: 8 January 2015 / Accepted: 27 March 2015 / Published: 2 April 2015 Abstract: In the present study, the feasibility to use phosphate solubilizing bacteria (PSB) to develop a biological leaching process of rare earth elements (REE) from monazite-bearing ore was determined. To predict the REE leaching capacity of bacteria, the phosphate solubilizing abilities of 10 species of PSB were determined by halo zone formation on Reyes minimal agar media supplemented with bromo cresol green together with a phosphate solubilization test in Reyes minimal liquid media as the screening studies. Calcium phosphate was used as a model mineral phosphate. Among the test PSB strains, Pseudomonas fluorescens, P. putida, P. rhizosphaerae, Mesorhizobium ciceri, Bacillus megaterium, and Acetobacter aceti formed halo zones, with the zone of A. -

COLUMBIUM - and RARE-EARTH ELEMENT-BEARING DEPOSITS at BOKAN MOUNTAIN, SOUTHEAST ALASKA by J
COLUMBIUM - AND RARE-EARTH ELEMENT-BEARING DEPOSITS AT BOKAN MOUNTAIN, SOUTHEAST ALASKA By J. Dean Warner and James C. Barker Alaska Field Operations Center * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * OFR - 33-89 UNITED STATES DEPARTMENT OF THE INTERIOR Manuel Lujan, Jr., Secretary BUREAU OF MINES T.S. Ary, Director TABLE OF CONTENTS Page Abstract ........................................................ 1 Introduction ....................... 2 Acknowledgments ................................................. 4 Location, access, and physiography .............................. 5 History and production ............. 5 Geologic setting .................................. 9 Trace element analyses of the peralkaline granite .............. 11 Nature of Bureau investigations ................................. 13 Sampling methods and analytical techniques .................... 13 Analytical interference, limitations, and self-shielding ...... 13 Resource estimation methods ................................... 15 Prospect evaluations .......................................... 16 Shear zones and fracture-controlled deposits .................... 18 Ross Adams mine ................. 18 Sunday Lake prospect.......................................... 21 I and L No. 1 and Wennie prospects ............................ 27 Other Occurrences ............................................. 31 Altered peralkaline granite ................................. 31 Dotson shear zone ........................................... 35 Resources .................................................. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse Et Al
US 2014O166788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse et al. (43) Pub. Date: Jun. 19, 2014 (54) METHOD AND SYSTEM FOR MAGNETIC (52) U.S. Cl. SEPARATION OF RARE EARTHIS CPC. B03C I/02 (2013.01); B02C 23/08 (2013.01); B02C23/20 (2013.01) (76) Inventors: Gary Pearse, Ottawa (CA); Jonathan USPC .......... 241/20: 209/212: 209/214; 241/24.14; Borduas, Montreal (CA); Thomas 241/21 Gervais, Montreal (CA); David Menard, Laval (CA); Djamel Seddaoui, Repentigny (CA); Bora Ung, Quebec (CA) (57) ABSTRACT (21) Appl. No.: 13/822,363 A system and a method for separating rare earth element (22) PCT Filed: Aug. 15, 2012 compounds from a slurry of mixed rare earth element com pounds, comprising flowing the slurry of mixed rare earth (86). PCT No.: PCT/CA12/50.552 element compounds through at least a first channel rigged S371 (c)(1), with at least a first magnet along a length thereof and con (2), (4) Date: May 17, 2013 nected to at least a first output channel at the position of the Publication Classification magnet, and retrieving individual rare earth element com pounds and/or groups of rare earth element compounds, sepa (51) Int. Cl. rated from the slurry as they are selectively attracted by the BO3C I/02 (2006.01) magnet and directed in the corresponding output channel B2C 23/20 (2006.01) according to their respective ratio of magnetic Susceptibility B2C 23/08 (2006.01) (AX) to specific density (Ap). Ferromagnetic particles are dia?pararnegnetic particles Submitted to a magnetic torque remain relatively unaffected by the torque As a result they roll left \\ Carried right by the slow rotating drum Patent Application Publication Jun. -

WO 2011/156817 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ t it o n 1 15 December 2011 (15.12.2011) WO 2011/156817 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C02F1/58 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US20 11/040214 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 13 June 201 1 (13.06.201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/354,031 11 June 2010 ( 11.06.2010) (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): MOLY- GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, CORP MINERALS LLC [US/US]; 561 Denver Tech ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, Center Pkwy, Suite 1000, Greenwood Village, CO 801 11 TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (US). -

Some Uses of Mischmetall in Organic Synthesis
REVIEW 9 Mischmetall in organic synthesis Organic synthesis Organic synthesis New from Acros Organics Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université de Paris-Sud, 91405, Orsay, France Some uses of mischmetall in organic synthesis ∗ Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université Paris-Sud, 91405, Orsay, France. Abstract: Mischmetall, an alloy of the light lanthanides, has been used in a variety of organic reactions, either as a coreductant in samarium(II)-mediated reactions (Barbier and Grignard-type reactions, pinacolic coupling reactions) or as the promoter of Reformatsky-type reactions. It has been also employed as the starting material for easy syntheses of lanthanide trihalides, the reactivity of which has been explored in Imamoto and Luche-Fukuzawa reactions and in Mukaiyama aldol reactions. Keywords: mischmetall, samarium, lanthanide, catalysis, organic reactions Introduction The use of rare earth compounds in organic chemistry has grown considerably during the past twenty years. Mainly samarium, cerium, lanthanum, ytterbium, neodymium, dysprosium, lutetium, scandium and yttrium metals and derivatives have been studied. These elements clearly differ in terms of reactivity. For instance, samarium(II) compounds can be used as powerful reductants whereas cerium(IV) ones are strong oxidants, some scandium and ytterbium(III) derivatives are very efficient catalysts in a variety of reactions and chemistry of lanthanum metal shows special features. We thought however that in many reactions, a mixture of lanthanide could be used instead of individual element without significant change in results. -

Infrare D Transmission Spectra of Carbonate Minerals
Infrare d Transmission Spectra of Carbonate Mineral s THE NATURAL HISTORY MUSEUM Infrare d Transmission Spectra of Carbonate Mineral s G. C. Jones Department of Mineralogy The Natural History Museum London, UK and B. Jackson Department of Geology Royal Museum of Scotland Edinburgh, UK A collaborative project of The Natural History Museum and National Museums of Scotland E3 SPRINGER-SCIENCE+BUSINESS MEDIA, B.V. Firs t editio n 1 993 © 1993 Springer Science+Business Media Dordrecht Originally published by Chapman & Hall in 1993 Softcover reprint of the hardcover 1st edition 1993 Typese t at the Natura l Histor y Museu m ISBN 978-94-010-4940-5 ISBN 978-94-011-2120-0 (eBook) DOI 10.1007/978-94-011-2120-0 Apar t fro m any fair dealin g for the purpose s of researc h or privat e study , or criticis m or review , as permitte d unde r the UK Copyrigh t Design s and Patent s Act , 1988, thi s publicatio n may not be reproduced , stored , or transmitted , in any for m or by any means , withou t the prio r permissio n in writin g of the publishers , or in the case of reprographi c reproductio n onl y in accordanc e wit h the term s of the licence s issue d by the Copyrigh t Licensin g Agenc y in the UK, or in accordanc e wit h the term s of licence s issue d by the appropriat e Reproductio n Right s Organizatio n outsid e the UK. Enquirie s concernin g reproductio n outsid e the term s state d here shoul d be sent to the publisher s at the Londo n addres s printe d on thi s page. -

Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and a Global Perspective
The Principal Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and a Global Perspective Gd Pr Ce Sm La Nd Scientific Investigations Report 2010–5220 U.S. Department of the Interior U.S. Geological Survey Cover photo: Powders of six rare earth elements oxides. Photograph by Peggy Greb, Agricultural Research Center of United States Department of Agriculture. The Principal Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and a Global Perspective By Keith R. Long, Bradley S. Van Gosen, Nora K. Foley, and Daniel Cordier Scientific Investigations Report 2010–5220 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2010 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This report has not been reviewed for stratigraphic nomenclature. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. Suggested citation: Long, K.R., Van Gosen, B.S., Foley, N.K., and Cordier, Daniel, 2010, The principal rare earth elements deposits of the United States—A summary of domestic deposits and a global perspective: U.S. -
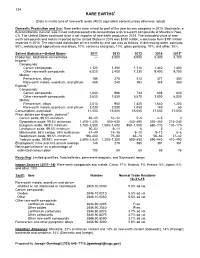
Mineral Commodity Summaries 2016
134 RARE EARTHS1 [Data in metric tons of rare-earth oxide (REO) equivalent content unless otherwise noted] Domestic Production and Use: Rare earths were mined for part of the year by one company in 2015. Bastnäsite, a fluorocarbonate mineral, was mined and processed into concentrates and rare-earth compounds at Mountain Pass, CA. The United States continued to be a net importer of rare-earth products in 2015. The estimated value of rare- earth compounds and metals imported by the United States in 2015 was $150 million, a decrease from $191 million imported in 2014. The estimated distribution of rare earths by end use was as follows, in decreasing order: catalysts, 60%; metallurgical applications and alloys, 10%; ceramics and glass, 10%; glass polishing, 10%; and other, 10%. Salient Statistics—United States: 2011 2012 2013 2014 2015e Production, bastnäsite concentrates — 3,000 5,500 5,400 4,100 Imports:2 Compounds: Cerium compounds 1,120 1,390 1,110 1,440 1,400 Other rare-earth compounds 6,020 3,400 7,330 9,400 9,700 Metals: Ferrocerium, alloys 186 276 313 371 360 Rare-earth metals, scandium, and yttrium 468 240 393 348 460 Exports:2 Compounds: Cerium compounds 1,640 996 734 608 600 Other rare-earth compounds 3,620 1,830 5,570 3,800 6,000 Metals: Ferrocerium, alloys 2,010 960 1,420 1,640 1,200 Rare-earth metals, scandium, and yttrium 3,030 2,080 1,050 140 60 Consumption, estimated 11,000 15,000 15,000 17,000 17,000 Price, dollars per kilogram, yearend:3 Cerium oxide, 99.5% minimum 40–45 10–12 5–6 4–5 2 Dysprosium oxide, 99.5% minimum 1,400–1,420 -

Bastnaesite Beneficiation by Froth Flotation and Gravity Separation
BASTNAESITE BENEFICIATION BY FROTH FLOTATION AND GRAVITY SEPARATION by Nathanael Williams A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Metallurgical and Materials Engineering). Golden, Colorado Date___________ Signed:_______________________ Nathanael Williams Date___________ Signed:_______________________ Dr. Corby Anderson Thesis Advisor Date:___________ Signed:_______________________ Dr. Angus Rockett Department Head Department of Metallurgical and Materials Engineering ii ABSTRACT Rare earth elements are in high demand in the United States. Independence from the importation of rare earths is essential to alleviate dependence on China for these rare earth elements. Bastnaesite, a rare earth fluorocarbonate, is one of the most abundant sources of rare earths in the United States. It is a fluorocarbonate mineral containing primarily cerium and lanthanum. The largest rare earth mine in the United States is Mountain Pass. This research was done to find a way to combine flotation with novel collectors and gravity separation techniques to reach an enhanced grade and recovery of rare earth elements while rejecting the gangue minerals, calcite, barite and silicate minerals. The main economic driving force is the price of hydrochloric acid in downstream processes, as calcite is an acid consumer. Surface chemistry analysis was completed using adsorption density, zeta potential, and microflotation on both gravity concentrates and run of mine ore samples. Four collectors were examined. These were N,2.dihydroxybenzamide, N-hydroxycyclohexanecarboxamide, N,3. dihydroxy-2.naphthamide, and N-hydroxyoleamide. Through this analysis it was determined that, to obtain the desired results, that flotation would be the rougher stage and the gravity separation would be utilized as the cleaner stage. -

ALPHABETICAL INDEX Names of Authors Are in Capitals, Subjects in Lower Case Roman, and Localities in Italics; Book Reviews Are Placed at the End
ALPHABETICAL INDEX Names of authors are in capitals, subjects in lower case roman, and localities in italics; book reviews are placed at the end. Accessory minerals, from Variscan granites of the Barometer, empirical calibration of the S valence Erzgebirge, Germany, 239 barometer, 421 Ag-Cu exchange, 399 Barstowite, crystal structure of, 901 Agate, defect structure and luminescence behaviour Bayan Obo Fe-Nb-REE deposit, Inner Mongolia, of, 149 stable isotope study of the carbonatitic dolomite Age determination, by instrument analysis, 297 host of the, 927 Agrostis capillaris, mineralogy of Pb-P grains in, 777 BELLATRECCIA,F., 123; zirconolite and calzirtite, 649 AIKAWA,N., 693 BENVENUTI,M., 111 A1 mass transfer, and diffusion in water, 633 BEYER, B. D., 735 Albite metasomatites, 519 BILAL, E., 567 Alkaline rocks, occurrence of several mineral phases BIRCH, W. D., 735 in, from Khaldzan Buregte and Tsakhir, 165 Bi-Sb energetics in sulphosalts and sulphides, 723 Allanite-(Ce) in granitic gneisses from the ultrahigh- Bismuthinite-stibnites, energetics in sulphosalts and pressure metamorphic terrane, Dabie ShaH, sulphides, 723 China, 579 Bone diagenesis, Palaeolithic, in the Arago cave at Alluvial diamonds, from Wellington, NSW, 447 Tautavel, France, 801 Almandine-spessartine-rich garnet, 503 Borate, crystal structure refinement of ludwigite, 511 Aluminosilicates, A1 mass transfer and diffusion in Bomite, exsolution behaviour in the bomite-digenite water, 633 series, 1 Aluminous efflorescences from the Te Kopia Braggite, Raman spectra of, 363 goethermal field, Taupo volcanic Zone, New Brazil, Curitiba, coutinite, discredited as identical to Zealand, 413 lanthanite-(La) and/or lanthanite-(Nd), 761; Alunogen, 413 Jacupiranga, crystal chemistry of zirconolite at-miargyrite, 399 and calzirtite from, 649 Analcime, synthesis of thallium-leucite pseudomorph BR/GATTI, M. -

Rare Earth Element and Yttrium Mineral Occurences in the Adirondack Mountains Marian V
6 RARE EARTH ELEMENT AND YTTRIUM MINERAL OCCURENCES IN THE ADIRONDACK MOUNTAINS MARIAN V. LUPULESCU,1 JEFFREY R. CHIARENZELLI,2 AND JARED SINGER3 1. Research and Collections, New York State Museum, Albany, NY 12230, [email protected] 2. Department of Geosciences, St. Lawrence University, Canton, NY 13617 3. Department of Earth and Environmental Sciences, Rensselaer Polytechnic Institute, Troy, NY 12180 KEYWORDS: Rare Earth Elements, Yttrium, Lyon Mountain Granite, Iron Deposits, Eastern Adirondack Highlands INTRODUCTION The rare earth elements (REE), formally called lanthanides, are a set of fifteen chemical elements, from lanthanum (La) to lutetium (Lu). Yttrium (Y) is added to this group because it has similar properties and occurs in the same mineral deposits. These elements are divided into low-atomic number, lanthanum to europium (La-Eu), or light rare earth elements (LREE), and heavy-atomic number, gadolinium to lutetium (Gd-Lu), or heavy rare earth elements (HREE). Even though they are called “rare,” they are widely distributed in the Earth’s crust and are present in all types of igneous, metamorphic, and sedimentary rocks. Some of the REE have abundances (relative amount of an element in the Earth’s crust) comparable or higher than gold, mercury, tungsten, tin, arsenic, copper, cobalt, and zinc (Handbook of Chemistry and Physics, 2016). In geology research, REE are used to understand earth processes such as crystal fractionation, partial melting, magma mixing, and absolute age dating of minerals. The REE have high-tech industrial applications and are essential for making hybrid cars, computers, smartphones, color TV, lasers, ceramics, and military devices. For this reason, the United States Geological Survey identified the rare earth element dysprosium as one of the most critical elements in the 2010 Critical Materials Strategy 80 THE ADIRONDACK JOURNAL OF ENVIRONMENTAL STUDIES VOLUME 21 81 6: RARE EARTH ELEMENT AND YTTRIUM MINERAL OCCURENCES IN THE ADIRONDACK MOUNTAINS report. -

Open Application of Pourbaix Diagrams
The Pennsylvania State University The Graduate School Department of Materials Science and Engineering APPLICATION OF POURBAIX DIAGRAMS IN THE HYDROMETALLURGICAL PROCESSING OF BASTNASITE A Thesis in Materials Science and Engineering by Isehaq S. Al-Nafai © 2015 Isehaq S. Al-Nafai Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science May 2015 i The thesis of Isehaq S. Al-Nafai was reviewed and approved* by the following: Kwadwo Osseo-Asare Distinguished Professor of Materials Science and Engineering, Metallurgy and Energy and Geo-Environmental Engineering. Thesis Adviser Hojong Kim Assistant Professor of Materials Science and Engineering Ismaila Dabo Assistant Professor of Materials Science and Engineering Suzanne Mohney Professor of Materials Science and Engineering and Electrical Engineering Chair, Intercollege Graduate Degree Program in Materials Science and Engineering *Signatures are on file in the Graduate School. ii ABSTRACT The hydrometallurgical processing of bastnasite was studied by constructing Pourbaix (potential vs. pH) diagrams at room temperature using HSC Chemistry 5.0 software. Different systems were considered to understand the overall behaviors of bastnasite and its species in aqueous systems. Most of the thermodynamic data were taken from HSC database, others were collected from literature and some were estimated. The standard Gibbs free energy of formation of bastnasite, REFCO3, were estimated using four different estimation methods. Each method shows its applicability, and the obtained average values were -379.9 kcal/mol for CeFCO3, -382.3 kcal/mol for LaFCO3, -379.8 kcal/mol for NdFCO3 and -381.3 kcal/mol for PrFCO3. RE-F-CO3-H2O systems show the stability regions of bastnasite which are located in nearly neutral to alkaline media (pH ~ 6.5-11).