Sarcomere Mechanics in Striated Muscles: from Molecules to Sarcomeres to Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microanatomy of Muscles
Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. Describe the 3 main types of muscles. 2. Detail the functions of the muscle system. 3. Correctly label the parts of a myocyte (muscle cell) 4. Identify the levels of organization in a skeletal muscle from organ to myosin. 5. Explain how a muscle contracts utilizing the correct terminology of the sliding filament theory. 6. Contrast and compare cardiac and smooth muscle with skeletal muscle. Major Functions: Muscle System 1. Moving the skeletal system and posture. 2. Passing food through the digestive system & constriction of other internal organs. 3. Production of body heat. 4. Pumping the blood throughout the body. 5. Communication - writing and verbal Specialized Cells (Myocytes) ~ Contractile Cells Can shorten along one or more planes because of specialized cell membrane (sarcolemma) and specialized cytoskeleton. Specialized Structures found in Myocytes Sarcolemma: The cell membrane of a muscle cell Transverse tubule: a tubular invagination of the sarcolemma of skeletal or cardiac muscle fibers that surrounds myofibrils; involved in transmitting the action potential from the sarcolemma to the interior of the myofibril. Sarcoplasmic Reticulum: The special type of smooth endoplasmic Myofibrils: reticulum found in smooth and a contractile fibril of skeletal muscle, composed striated muscle fibers whose function mainly of actin and myosin is to store and release calcium ions. Multiple Nuclei (skeletal) & many mitochondria Skeletal Muscle - Microscopic Anatomy A whole skeletal muscle (such as the biceps brachii) is considered an organ of the muscular system. Each organ consists of skeletal muscle tissue, connective tissue, nerve tissue, and blood or vascular tissue. -

The Muscular System
THE MUSCULAR SYSTEM COMPILED BY HOWIE BAUM 1 Muscles make up the bulk of the body and account for 1/3 of its weight.!! Blood vessels and nerves run to every muscle, helping control and regulate each muscle’s function. The muscular system creates body heat and also moves the: Bones of the Skeletal system Food through Digestive system Blood through the Circulatory system Fluids through the Excretory system MUSCLE TISSUE The body has 3 main types of muscle tissue 1) Skeletal, 2) Smooth, and 3) Cardiac SKELETAL MUSCLE SMOOTH MUSCLE CARDIAC MUSCLE Skeletal muscles attach to and move bones by contracting and relaxing in response to voluntary messages from the nervous system. Skeletal muscle tissue is composed of long cells called muscle fibers that have a striated appearance. Muscle fibers are organized into bundles supplied by blood vessels and innervated by motor neurons. Muscle structure Skeletal (striated or voluntary) muscle consists of densely packed groups of hugely elongated cells known as myofibers. These are grouped into bundles (fascicles). A typical myofiber is 2–3 centimeters ( 3/4–1 1/5 in) long and 0.05millimeters (1/500 inch) in diameter and is composed of narrower structures – myofibrils. These contain thick and thin myofilaments made up mainly of the proteins actin and myosin. Numerous capillaries keep the muscle supplied with the oxygen and glucose needed to fuel contraction. Skeletal Muscles • Skeletal muscles attach to bones by tendons (connective tissue) and enable movement. • Skeletal muscles are mostly voluntary Feel the back of your ankle to feel your Achilles tendon - the largest tendon in your body. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics
International Journal of Molecular Sciences Review Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics Martijn van de Locht , Tamara C. Borsboom, Josine M. Winter and Coen A. C. Ottenheijm * Department of Physiology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Location VUmc, 1081 HZ Amsterdam, The Netherlands; [email protected] (M.v.d.L.); [email protected] (T.C.B.); [email protected] (J.M.W.) * Correspondence: [email protected]; Tel.: +31-(0)-20-444-8123 Abstract: The troponin complex is a key regulator of muscle contraction. Multiple variants in skeletal troponin encoding genes result in congenital myopathies. TNNC2 has been implicated in a novel congenital myopathy, TNNI2 and TNNT3 in distal arthrogryposis (DA), and TNNT1 and TNNT3 in nemaline myopathy (NEM). Variants in skeletal troponin encoding genes compromise sarcomere function, e.g., by altering the Ca2+ sensitivity of force or by inducing atrophy. Several potential therapeutic strategies are available to counter the effects of variants, such as troponin activators, introduction of wild-type protein through AAV gene therapy, and myosin modulation to improve muscle contraction. The mechanisms underlying the pathophysiological effects of the variants in skeletal troponin encoding genes are incompletely understood. Furthermore, limited knowledge is available on the structure of skeletal troponin. This review focusses on the physiology of slow and fast skeletal troponin and the pathophysiology of reported variants in skeletal troponin encoding genes. A better understanding of the pathophysiological effects of these variants, together with enhanced knowledge regarding the structure of slow and fast skeletal troponin, will direct the development of Citation: van de Locht, M.; treatment strategies. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
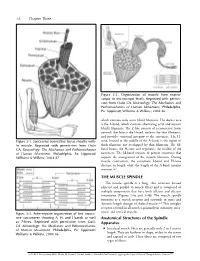
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells
Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells JOHN W. KRUEGER, DAMIAN FORLETTI, and BEATRICE A. WITTENBERG From the Albert Einstein College of Medicine, Departments of Medicine and Physiology, Bronx, New York 10461 ABSTRACT We have observed the dynamics of sarcomere shortening and the diffracting action of single, functionally intact, unattached cardiac muscle cells enzymatically isolated from the ventricular tissue of adult rats. Sarcomere length was measured either (a) continuously by a light diffraction method or (b) by direct inspection of the cell's striated image as recorded on videotape or by cinemicroscopy (120-400 frames/s). At physiological levels of added CaCl2 (0.5- 2.0 mM), many cells were quiescent (i.e., they did not beat spontaneously) and contracted in response to electrical stimulation (~ 1.0-ms pulse width). Sarcomere length in the quiescent, unstimulated cells (1.93 + 0.10 [SD] /~m), at peak shortening (1.57 + 0.13/~m, n = 49), and the maximum velocity of sarcomere shortening and relengthening were comparable to previous observations in intact heart muscle preparations. The dispersion of light diffracted by the cell remained narrow, and individual striations remained distinct and laterally well registered throughout the shortening-relengthening cycle. In contrast, apprecia- ble nonuniformity and internal buckling were seen at sarcomere lengths < 1.8 /~m when the resting cell, embedded in gelatin, was longitudinally compressed. These results indicate (a) that shortening and relengthening is characterized by uniform activation between myofibrils within the cardiac cell and (b) that physiologically significant relengthening forces in living heart muscle originate at the level of the cell rather than in extracellular connections. -

Laboratory Methodology for the Histological Study of Skeletal Muscle
ReviewArtículo original Laboratory methodology for the histological study of skeletal muscle Fernando Leiva-Cepas1,2,3, Ignacio Ruz-Caracuel1,2*, María A. Peña-Toledo2,3, Antonio Agüera-Vega1,2, Ignacio Jimena1,2,3, Evelio Luque1,3, José Peña1,2,3 1Departamento de Ciencias Morfológicas. Universidad de Córdoba. Córdoba. 2Grupo de Investigación en Regeneración Muscular. Universidad de Córdoba. 3Instituto Maimónides de Investigación Biomédica de Córdoba. IMIBIC. Córdoba. *Servicio de Anatomía Patológica. Hospital Universitario La Paz, IDIPAZ, Madrid. Received: 11.12.2017 Summary Accepted: 15.02.2018 Skeletal muscle is a malleable and dynamic tissue capable of a high degree of plasticity in regards to its histological confi- guration. In this sense, microscopic study is an important and essential tool for the analysis of adaptive processes -such as hypertrophy or changes of fiber types- and the regeneration or repair of skeletal muscle after injury, in the fields of sports medicine and traumatology respectively. While light microscopy addresses the study of the different constitutive elements into the skeletal muscle and their relationships with each other that determine the organ histoarchitecture, with electron microscopy an ultrastructural analysis is carried out that allows to relate the structure and function of the individual cells. This article illustrates a pragmatic and practical approach, based on personal experience and a review of the literature, from the conditions in obtaining and sending samples of skeletal muscle to the laboratory to the procedures to prepare them for histological study (sections of cryostat, paraffin sections and electron microscopy). Especially we focus on the description of Key words: the processing by freezing and recommendations to follow, as this is the ideal method for this tissue. -

Identification of the Calmodulin-Bindingdomain Of
Proc. Nati. Acad. Sci. USA Vol. 82, pp. 3187-3191, May 1985 Biochemistry Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase (protein phosphorylation/Ca2+-dependent enzyme regulation/synthetic peptide) DONALD K. BLUMENTHAL*t, Koji TAKIO*t, ARTHUR M. EDELMAN*t, HARRY CHARBONNEAUt, KOITI TITANIt, KENNETH A. WALSHt, AND EDWIN G. KREBS*tt *Howard Hughes Medical Institute, tDepartment of Pharmacology, and tDepartment of Biochemistry, University of Washington School of Medicine, Seattle, WA 98195 Contributed by Edwin G. Krebs, January 28, 1985 ABSTRACT In the course of determining the primary modulin-dependent enzyme structure. One of the goals in structure of rabbit skeletal muscle myosin light chain kinase this endeavor was to identify the calmodulin-binding domain (MLCK; ATP:protein phosphotransferase, EC 2.7.1.37) a of this enzyme. The sequence and preliminary characteriza- peptide fragment was obtained that appears to represent the tion of a peptide derived from MLCK that displays the prop- calmodulin-binding domain of this enzyme. Low concentra- erties expected of a calmodulin-binding domain are de- tions of the peptide inhibited calmodulin activation of MLCK scribed in this report. This information should prove useful (Ki 1 nM). The peptide was not associated with a catalytical- in understanding the evolutionary relationships of calmodu- ly active, calmodulin-independent form of MLCK that was ob- lin-dependent enzymes as well as the molecular mechanisms tained by limited proteolysis. The peptide is 27 residues in of calmodulin action. length and represents the carboxyl terminus of MLCK. The sequence of the peptide shows no significant homology with METHODS any known protein sequence. -

Spinal Reflexes
Spinal Reflexes Lu Chen, Ph.D. MCB, UC Berkeley 1 Simple reflexes such as stretch reflex require coordinated contraction and relaxation of different muscle groups Categories of Muscle Based on Direction of Motion Flexors Æ reduce the angle of joints Extensors Æ increase the angle of joints Categories of Muscle Based on Movement Agonist Æmuscle that serves to move the joint in the same direction as the studied muscle Antagonist Æ muscle that moves the joint in the opposite direction 2 1 Muscle Spindles •Small encapsulated sensory receptors that have a Intrafusal muscle spindle-like shape and are located within the fibers fleshy part of the muscle •In parallel with the muscle fibers capsule •Does not contribute to the overall contractile Sensory force endings •Mechanoreceptors are activated by stretch of the central region Afferent axons •Due to stretch of the whole muscle Efferent axons (including intrafusal f.) •Due to contraction of the polar regions of Gamma motor the intrafusal fibers endings 3 Muscle Spindles Organization 2 kinds of intrafusal muscle fibers •Nuclear bag fibers (2-3) •Dynamic •Static •Nuclear chain fibers (~5) •Static 2 types of sensory fibers •Ia (primary) - central region of all intrafusal fibers •II (secondary) - adjacent to the central region of static nuclear bag fibers and nuclear chain fibers Intrafusal fibers stretched Sensory ending stretched, (loading the spindle) increase firing Muscle fibers lengthens Sensory ending stretched, (stretched) increase firing Spindle unloaded, Muscle fiber shortens decrease firing 4 2 Muscle Spindles Organization Gamma motor neurons innervate the intrafusal muscle fibers. Activation of Shortening of the polar regions gamma neurons of the intrafusal fibers Stretches the noncontractile Increase firing of the center regions sensory endings Therefore, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere.