Ocular Motor Nerve Development in the Presence and Absence of Extraocular Muscle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens)
Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens) Lecture (13) ▪ Important ▪ Doctors Notes Please check our Editing File ▪ Notes/Extra explanation ه هذا العمل مب ين بشكل أسا يس عىل عمل دفعة 436 مع المراجعة { َوَم نْ يَ َت َو َ ّكْ عَ َلْ ا َّْلل فَهُ َوْ َحْ سْ ُ ُُْ} والتدقيق وإضافة المﻻحظات وﻻ يغ ين عن المصدر اﻷسا يس للمذاكرة ▪ Objectives At the end of the lecture, students should be able to: ✓ List the cranial nuclei related to occulomotor, trochlear, and abducent nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the site of emergence and course of these 3 nerves. ✓ Describe the important relations of oculomotor, trochlear, and abducent nerves in the orbit ✓ List the orbital muscles supplied by each of these 3 nerves. ✓ Describe the effect of lesion of each of these 3 nerves. ✓ Describe the optic nerve and visual pathway. Recall the how these nerves exit from the brain stem: Optic (does not exit from brain stem) Occulomotor: ventral midbrain (medial aspect of crus cerebri) Trochlear: dorsal midbrain (caudal to inferior colliculus) Abducent: ventral Pons (junction b/w pons & pyramid) Brain (Ventral view) Brain stem (Lateral view) Extra-Ocular Muscles 7 muscles: (ترفع جفن العين) .Levator palpebrae superioris 1- Origin: from the roof of the orbit (4) Recti muscles: *Rectus: ماشي على ( Superior rectus (upward and medially 2- الصراط (Inferior rectus (downward and medially 3- المستقيم 4- Medial rectus (medial) (medial) 5- Lateral rectus (lateral) How to remember the 2 فحركته muscles not supplied by نفس اسمه -اسمها عكس وظيفتها- :Oblique muscles (2) 6- Superior oblique (downward and laterally) Oblique: CN3? Superior oblique goes -1 منحرفOrigin: from the roof of the orbit 7- Inferior oblique (upward and laterally) up (superior) and turns around (oblique) a notch يمشي Origin: from the anterior floor or pulley and its supply is عكس كﻻمه NB. -

The Accessory Optic System: Basic Organization with an Update on Connectivity, Neurochemistry, and Function
UC Irvine UC Irvine Previously Published Works Title The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function. Permalink https://escholarship.org/uc/item/3v25z604 Journal Progress in brain research, 151 ISSN 0079-6123 Authors Giolli, Roland A Blanks, Robert H I Lui, Fausta Publication Date 2005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Chapter 13 The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function Roland A. Giolli1, , , Robert H.I. Blanks1, 2 and Fausta Lui3 1Department of Anatomy and Neurobiology, University of California, College of Medicine, Irvine, CA 92697, USA 2Charles E. Schmidt College of Science, Florida Atlantic University, 777 Glades Rd., P.O. Box 3091, Boca Raton, FL 33431, USA 3Dipartimento di Scienze Biomediche, Sezione di Fisiologia, Universita di Modena e Reggio Emilia, Via Campi 287, 41100, Modena, Italy Available online 10 October 2005. Abstract The accessory optic system (AOS) is formed by a series of terminal nuclei receiving direct visual information from the retina via one or more accessory optic tracts. In addition to the retinal input, derived from ganglion cells that characteristically have large receptive fields, are direction-selective, and have a preference for slow moving stimuli, there are now well-characterized afferent connections with a key pretectal nucleus (nucleus of the optic tract) and the ventral lateral geniculate nucleus. The efferent connections of the AOS are robust, targeting brainstem and other structures in support of visual-oculomotor events such as optokinetic nystagmus and visual–vestibular interaction. This chapter reviews the newer experimental findings while including older data concerning the structural and functional organization of the AOS. -

Isolated Superior Rectus Palsy Due to Contralateral Midbrain Infarction
OBSERVATION Isolated Superior Rectus Palsy Due to Contralateral Midbrain Infarction Jee-Hyun Kwon, MD; Sun U. Kwon, MD; Hyo-Sook Ahn, MD; Ki-Bum Sung, MD; Jong S. Kim, MD Background: Isolated superior rectus palsy due imaging showed a tiny infarct at the area of the oculo- to a contralateral midbrain lesion has not been re- motor nucleus on the contralateral side. ported. Conclusion: Isolated superior rectus palsy may be caused by a contralateral midbrain lesion that selec- Case Description: A 71-year-old woman suddenly tively involves crossing superior rectus nerve fibers. developed diplopia. Examination showed that she had isolated superior rectus paresis. Magnetic resonance Arch Neurol. 2003;60:1633-1635 IDBRAIN INFARCTS may the red glass test, maximally separated im- produce ocular motor ages were present on the right, upward paresis without other gaze when the red image was present su- neurological signs.1 perior to the white one. Weakness of a single Although the palpebral fissure in the Mextraocular muscle has also been re- right eye appeared slightly narrow as com- ported to be caused by a small midbrain pared with the left one, the patient and her infarction.1 However, to our knowledge, relatives stated that this had been present isolated contralateral superior rectus palsy long before hospital admission. A fundus ex- had not been reported to be caused by mid- amination showed extorsion of the right eye brain lesions. without a torsional component in the left eye (Figure 1B). Diplopia test findings with REPORT OF A CASE the Hess chart were consistent with the su- perior rectus palsy in the right eye. -

Cranial Nerves and Their Nuclei
CranialCranial nervesnerves andand theirtheir nucleinuclei 鄭海倫鄭海倫 整理整理 Cranial Nerves Figure 13.4a Location of the cranial nerves • Anterior cranial fossa: C.N. 1–2 • Middle cranial fossa: C.N. 3-6 • Posterior cranial fossa: C.N. 7-12 FunctionalFunctional componentscomponents inin nervesnerves • General Somatic Efferent • Special Visceral Afferent •GSE GSA GVE GVA • (SSE) SSA SVE SVA Neuron columns in the embryonic spinal cord * The floor of the 4th ventricle in the embryonic rhombencephalon Sp: special sensory B:branchial motor Ss: somatic sensory Sm: somataic motor Vi: visceral sensory A: preganglionic autonomic (visceral motor) • STT: spinothalamic tract • CST: corticospinal tract • ML: medial lemniscus Sensory nerve • Olfactory (1) •Optic (2) • Vestibulocochlear (8) Motor nerve • Oculomotor (3) • Trochlear (4) • Abducens (6) • Accessory (11) • Hypoglossal (12) Mixed nerve • Trigeminal (5) • Facial (7) • Glossopharyngeal (9) • Vagus (10) Innervation of branchial muscles • Trigemial • Facial • Glossopharyngeal • Vagus Cranial Nerve I: Olfactory Table 13.2(I) Cranial Nerve II: Optic • Arises from the retina of the eye • Optic nerves pass through the optic canals and converge at the optic chiasm • They continue to the thalamus (lateral geniculate body) where they synapse • From there, the optic radiation fibers run to the visual cortex (area 17) • Functions solely by carrying afferent impulses for vision Cranial Nerve II: Optic Table 13.2(II) Cranial Nerve III: Oculomotor • Fibers extend from the ventral midbrain, pass through the superior orbital fissure, and go to the extrinsic eye muscles • Functions in raising the eyelid, directing the eyeball, constricting the iris, and controlling lens shape Cranial Nerve III: Oculomotor Table 13.2(III) 1.Oculomotor nucleus (GSE) • Motor to ocular muscles: rectus (superior對側, inferior同側and medial同 側),inferior oblique同側, levator palpebrae superioris雙側 2. -

Urgency, Emergency, Or 'Prefergency'
Urgency, Emergency, or ‘Prefergency’ The Basics of Clinically Relevant Ophthalmology April 28, 2019 CVMA – SBCV Spring Seminar This four-hour seminar will be broken up in to three parts. Part 1 will briefly review the pupillary light reflex. Part 2 will briefly review tonometry, and Part 3 will be broken into several case-based examples of how the PLR, tonometry can help to best manage your cases in your own practice. The Pupillary Light Reflex The pupillary light reflex (PLR) is a grossly underutilized tool that can be performed on every patient with minimal equipment or time. Using only a bright focal light source (eg pen light) and a dim room, stimulation of the retina with light begins a circuit of electric synapses that when intact, manifests as pupil constriction of both the stimulated eye and the fellow eye. Failure to constrict the pupil of either eye indicates a break in an otherwise intact neurologic circuit. When combined with vision reflex and response testing, a defect in the unobservable electrical circuit made up of cranial nerves and brain can be localized. Fig 1. Diagram showing the intracranial pathway of the PLR is likely a shuddering memory from veterinary school. The drawings were always complicated, easily confused, and quickly forgotten. Happily, the pupillary light reflex is really quite simple and will be summarized below. Specific cases will be presented to help refine the importance of the PLR in every case being evaluated. 1 There are two main parts of the PLR: the afferent and efferent pathways. Each pathway can be divided into four parts with a single deviation point between the two. -

Cranial Nerves II, III, IV & VI
Cranial Nerves II, III, IV & VI Neuroanatomy block-Anatomy-Lecture 13 Editing file Objectives At the end of the lecture, students should be able to: 01 List the cranial nuclei related to occulomotor, trochlear, and abducent nerves in the brain stem. 02 Describe the type and site of each nucleus. 03 Describe the site of emergence and course of these 3 nerves. 04 Describe the important relations of occulomotor, trochlear, and abducent nerves in the orbit. 05 List the orbital muscles supplied by each of these 3 nerves. Color guide 06 Describe the effect of lesion of each of these 3 nerves. ● Only in boys slides in Green ● Only in girls slides in Purple 07 Describe the optic nerve and visual pathway. ● important in Red ● Notes in Grey Extra-Ocular Muscles 7 muscles 1. Levator palpebrae superioris. (Elevates the upper eyelid) 2. Medial rectus.(medial) 3. Lateral rectus.(lateral) 4. Superior rectus.(upward and medially ) 5. Inferior rectus.(downward and medially) 6. Superior oblique.(downward and laterally) 7. Inferior oblique.(upward and laterally) ❖ All muscles of the eye are supplied by the oculomotor nerve, EXCEPT LR6 lateral rectus (by abducens) + SO4 superior oblique (by trochlear) Brain stem (Lateral view) Brain (Ventral view) 3 Oculomotor Nerve ➢ Motor for most of extraocular muscles. ➢ Also carries preganglionic parasympathetic fibers to the pupillary constrictor (pupillary reflex) and ciliary muscles (accomodation). Has two nuclei Accessory nucleus Main oculomotor nucleus (Edinger-Westphal nucleus) ❖ Lies in the midbrain at the ❖ Lies dorsal to the main motor level of superior colliculus, nucleus. ❖ located in the periaqueductal ❖ receives Fibres from the pretectal grey matter. -

Brainstem II
Brainstem II Medical Neuroscience Dr. Wiegand Internal Brainstem | Cranial nerve nuclei | Location of selected tracts | Reticular formation Developmental Organization 1 Developmental Organization Sulcus Limitans Developmental Organization FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-14-1 2 Cranial Nerve Nuclei Organization | Medial to sulcus limitans z GSE ⇒ SVE ⇒ GVE | Lateral from sulcus limitans z VA ⇒ GSA ⇒ SSA FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-44-4 SEN MOT Generalizations | Sensory nuclei lateral to sulcus limitans | Motor nuclei medial to sulcus limitans | Visceral nuclei are on either side of sulcus | Innervation of skeletal muscle (GSE & SVE) most medial | General and special visceral afferent nuclei in same column I, II Cranial Nerves – Telencephalon & Diencephalon | Olfactory – z smell (SVA) | Optic – z vision (SSA) 3 III, IV Cranial Nerves – Mesencephalon | Oculomotor – z extraocular eye muscles (GSE) – oculomotor nucleus z PSNS to eye (GVE) – Edinger-Westphal nucleus | Trochlear – z extraocular muscle (sup. oblique) (GSE) – trochlear nucleus V, VI Cranial Nerves – Metencephalon | Trigeminal – z Masticatory muscles (SVE) – trigeminal motor nucleus z General sensation of the head and face (GSA) – trigeminal complex | Abducens – z extraocular muscle (lat. rectus) (GSE) – abducens nucleus VII Cranial Nerves – Metencephalon | Facial – z Facial expression muscles (SVE) – facial motor nucleus z Glands (submandibular, sublingual & lacrimal) (GVE) – superior salivatory & lacrimal nucleus z Taste (SVA) – rostral solitary nucleus z General sensation of ear (GSA) – trigeminal complex 4 VIII Cranial Nerves – Metencephalon Vestibulocochlear – z Hearing (SSA) – dorsal and ventral cochlear nuclei z Balance (SSA) – vestibular nuclei IX Cranial Nerves – Mylencephalon | Glossopharyngeal z Stylopharyngeus muscle (SVE) – n. ambiguus z PSNS to parotid gland (GVE) – inferior salivatory n. z Taste (SVA) – rostral solitary n. -

Full Disclosures
RESIDENT & FELLOW SECTION Pearls & Oy-sters: Section Editor Looking up the anatomy of looking up John J. Millichap, MD Muhammad Bilal Abid, PEARLS complex ophthalmoplegia by the admitting emer- MBBS 1. A unilaterally dilated pupil would indicate that the gency physicians. Derek Soon, MBBS lesion is fascicular rather than nuclear as the MRI revealed a lesion in the anterior periaqueduc- Rahul Rathakrishnan, BM Edinger-Westphal nucleus is in the midline and tal midbrain, eccentrically situated to the left of the Paul Zhao, MBBS causes bilateral mydriasis. midline. The apparent diffusion coefficient character- Clement Tan, MBBS 2. The inferior rectus, medial rectus, and inferior istics were in keeping with an acute infarct (figure 1, Leonard L.L. Yeo, MBBS oblique muscles are supplied ipsilaterally while A and B) and magnetic resonance angiogram did not the superior rectus is supplied contralaterally. reveal any stenosis. The patient was treated with aspi- rin, statins, and tight blood pressure control. Correspondence to Dr. Yeo: OY-STERS DISCUSSION [email protected] In our patient, abduction, adduction, 1. Parinaud syndrome is classically from a compres- downgaze, and convergence were preserved, and there sive lesion on the dorsal midbrain; however, focal was no ptosis, indicating that the respective subnuclei lesions can interrupt the pathways and result in were spared. Apart from esophoria and a pseudo-6th similar features. nerve palsy, the only findings were asymmetric upgaze 2. If the presentation is explainable by a single lesion restriction and lid retraction (figure 2A and video). in the oculomotor complex, investigations for This can be explained by the infarct in the caudal causes such as Miller Fisher syndrome and myas- midline region in the posterior commissure location thenia gravis may be avoidable. -

Lab 3. Pons & Midbrain
Lab 3. Pons & Midbrain Lesion Lessons Lesion 4.1 Anne T. Pasta i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; E.C. Alvord, Jr., Univ. of Washington) iii) Cause: Lesion 4.2 Colin S. Terase i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; M.Z. Jones, Michigan St. Univ.) iii) Cause: Medical Neuroscience 4– Pontine Level of the Facial Genu Locate and note the following: Basilar pons – massive ventral structure provides the most obvious change from previous med- ullary levels. Question classic • pontine gray - large nuclear groups in the basilar pons. Is the middle cerebellar peduncle composed – origin of the middle cerebellar peduncle of climbing or mossy • pontocerebellar axons - originate from pontine gray neurons and cross to form the fibers? middle cerebellar peduncle. • corticopontine axons- huge projection that terminates in the basilar pontine gray. • corticospinal tract axons – large bundles of axons surrounded by the basilar pontine gray. – course caudally to form the pyramids in the medulla. Pontine tegmentum • medial lemniscus - has now assumed a “horizontal” position and forms part of the border between the basilar pons and pontine tegmentum. Question classic • central tegmental tract - located just dorsally to the medial lemniscus. What sensory modali- – descends from the midbrain to the inferior olive. ties are carried by the • superior olivary nucleus - pale staining area lateral to the central tegmental tract. medial and lateral – gives rise to the efferent olivocochlear projection to the inner ear. lemnisci? • lateral lemniscus - lateral to the medial lemniscus. – composed of secondary auditory projections from the cochlear nuclei. -
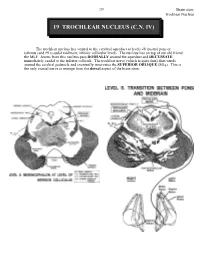
19 Trochlear Nucleus (C.N
299 Brain stem Trochlear Nucleus 19 TROCHLEAR NUCLEUS (C.N. IV) The trochlear nucleus lies ventral to the cerebral aqueduct at levels #8 (rostral pons or isthmus) and #9 (caudal midbrain; inferior collicular level). The nucleus lies on top of our old friend the MLF. Axons from this nucleus pass DORSALLY around the aqueduct and DECUSSATE immediately caudal to the inferior colliculi. The trochlear nerve (which is quite thin) then winds around the cerebral peduncle and eventually innervates the SUPERIOR OBLIQUE (SO4). This is the only cranial nerve to emerge from the dorsal aspect of the brain stem. Brain stem 300 Trochlear nucleus While at first glance it appears that contraction of the superior oblique turns the eye down and out, the rest of the story (Paul Harvey would love this) is slightly more complicated. If you are interested, read on! The vector diagram resolves the arrow R-B into effective components. Vector R-A depresses the eye around the lateral axis. Vector R-C abducts the eye around the vertical axis and intorts (medial rotation) the eye around the anteroposterior axis. Therefore, vector R-B acts to depress, abduct and intort the eye. When the eye is in the primary position, the superior oblique lies medial to the A-P axis of the globe. However, when the eye is adducted, the line of pull of the tendon of the superior oblique is parallel to the A-P axis of the globe. In this position, none of the actions of the muscle are dissipated in the other actions (abduction and intorsion). -
The Localization and Complete Extent of the Newly Defined Preganglionic
Aus der Anatomischen Anstalt der Ludwig-Maximilians-Universität München Lehrstuhl I - Vegetative Anatomie Vorstand: Prof. Dr. med. Jens Waschke The localization and complete extent of the newly defined preganglionic Edinger-Westphal nucleus (EWpg) in human Dissertation zum Erwerb des Doktorgrades der Medizin an der Medizinischen Fakultät der Ludwig-Maximilians-Universität zu München vorgelegt von Laura Ioana Valeanu aus Sibiu, Rumänien Jahr 2020 1 Mit Genehmigung der Medizinischen Fakultät der Universität München Berichterstatter: Prof. Dr. med. Jens Waschke Mitberichterstatter: Prof. Dr. Stefan Glasauer PD Dr. Christoph Trumm Mitbetreuung durch die promovierte Mitarbeiterin: Prof. Dr. rer. nat. Anja Horn-Bochtler Dekan: Prof. Dr. med. dent. Reinhard Hickel Tag der mündlichen Prüfung: 19.05.2020 2 Table of contents ABSTRACT ........................................................................................................................................... 8 ZUSAMMENFASSUNG ...................................................................................................................... 9 I. INTRODUCTION ............................................................................................................................. 11 I.1. Anatomy and function of the inner eye muscles .......................................................... 11 I.1.1. The iris sphincter muscle ................................................................................................. 11 I.1.2. The ciliary muscle ............................................................................................................ -
Activated K Channels in Parasympathetic Neurons
-Neuregulin-1 is required for the in vivo development of functional Ca2؉-activated K؉ channels in parasympathetic neurons Jill S. Cameron, Laurence Dryer, and Stuart E. Dryer* Department of Biology and Biochemistry, University of Houston, Houston, TX 77204-5513 Edited by Eric M. Shooter, Stanford University School of Medicine, Stanford, CA, and approved December 21, 2000 (received for review August 17, 2000) 2؉ ؉ The development of functional Ca -activated K channels (KCa)in are expressed in the chick Edinger–Westphal nucleus, the source chick ciliary ganglion (CG) neurons requires interactions with of midbrain preganglionic neurons that innervate the CG (20). afferent preganglionic nerve terminals. Here we show that the Second, functional erbB receptors are expressed in chick CG essential preganglionic differentiation factor is an isoform of neurons (20). Finally, application of recombinant -NEU1 can   -neuregulin-1. -Neuregulin-1 transcripts are expressed in the increase the density of functional KCa in cultured CG neurons, midbrain preganglionic Edinger–Westphal nucleus at developmen- at least at high concentrations (21). However, this effect also can tal stages that coincide with or precede the normal onset of be evoked by high concentrations of other erbB ligands such as  macroscopic KCa in CG neurons. Injection of -neuregulin-1 peptide EGF and transforming growth factor ␣ (TGF␣), but not by into the brains of developing embryos evoked a robust stimulation ␣-neuregulin (21). In addition, it is not known whether -Neu1 of functional KCa channels at stages before the normal appearance transcripts are expressed in the Edinger–Westphal nucleus at of these channels in CG neurons developing in vivo.