Handbook of Waterfowl Behavior: Summary and Synopsis of the Family Anatidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Structure of Taiga Bean Goose in Central Scandinavia
Bird Conservation International, page 1 of 14. © BirdLife International, 2018 doi:10.1017/S0959270918000205 Birds of different feather flock together - genetic structure of Taiga Bean Goose in Central Scandinavia ADRIAAN DE JONG, ODDMUND KLEVEN, JAN EIVIND ØSTNES, ROLF TERJE KROGLUND, ISAK VAHLSTRÖM, JAN NILSSON and GÖRAN SPONG Summary During their flightless summer moult, Taiga Bean Geese Anser fabalis fabalis gather at commu- nal moulting sites. Individuals from the Nord-Trøndelag breeding area in Norway have been observed to join with local individuals on moulting sites in Vilhelmina Municipality, Sweden. These two groups show distinct features in breeding habitat and migratory behaviour, but are they also genetically distinct? We used 12 microsatellite loci for genotyping 109 blood, feather and faecal samples from three sampling areas (Røyrvik in Norway and Stalon and Nästansjö in Sweden) to examine genetic diversity and structure. Clustering and Principal Coordinate analyses of all samples unveiled at least two distinct clusters, which were unevenly distributed over the sampling sites. Grouped by sampling sites, AMOVA and FST analyses showed that samples from the three sites differed genetically. These differences were larger between Røyrvik and Nästansjö than between Stalon and the other two. Relatedness was high among the Røyrvik samples. From our results we conclude that one of the clusters describes the Røyrvik breeding subpopulation, while the other(s) breed mainly in Sweden. Although these subpopulations simultaneously use the same moulting area in Vilhelmina, they appear to be ecologically, behaviourally and genetically distinct, in particular the Røyrvik sub-population. For goose conservation and management, we suggest that the Nord-Trøndelag (Røyrvik) subpopulation is considered a separate flyway man- agement unit. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

An Introduction to the Bofedales of the Peruvian High Andes
An introduction to the bofedales of the Peruvian High Andes M.S. Maldonado Fonkén International Mire Conservation Group, Lima, Peru _______________________________________________________________________________________ SUMMARY In Peru, the term “bofedales” is used to describe areas of wetland vegetation that may have underlying peat layers. These areas are a key resource for traditional land management at high altitude. Because they retain water in the upper basins of the cordillera, they are important sources of water and forage for domesticated livestock as well as biodiversity hotspots. This article is based on more than six years’ work on bofedales in several regions of Peru. The concept of bofedal is introduced, the typical plant communities are identified and the associated wild mammals, birds and amphibians are described. Also, the most recent studies of peat and carbon storage in bofedales are reviewed. Traditional land use since prehispanic times has involved the management of water and livestock, both of which are essential for maintenance of these ecosystems. The status of bofedales in Peruvian legislation and their representation in natural protected areas and Ramsar sites is outlined. Finally, the main threats to their conservation (overgrazing, peat extraction, mining and development of infrastructure) are identified. KEY WORDS: cushion bog, high-altitude peat; land management; Peru; tropical peatland; wetland _______________________________________________________________________________________ INTRODUCTION organic soil or peat and a year-round green appearance which contrasts with the yellow of the The Tropical Andes Cordillera has a complex drier land that surrounds them. This contrast is geography and varied climatic conditions, which especially striking in the xerophytic puna. Bofedales support an enormous heterogeneity of ecosystems are also called “oconales” in several parts of the and high biodiversity (Sagástegui et al. -

Wetlands Australia
Wetlands Australia National wetlands update August 2014—Issue No 25 Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for the Environment. © Copyright Commonwealth of Australia, 2014 Wetlands Australia National Wetlands Update August 2014 – Issue No 25 is licensed by the Commonwealth of Australia for use under a Creative Commons By Attribution 3.0 Australia licence with the exception of the Coat of Arms of the Commonwealth of Australia, the logo of the agency responsible for publishing the report, content supplied by third parties, and any images depicting people. For licence conditions see: http://creativecommons.org/licenses/by/3.0/au This report should be attributed as ‘Wetlands Australia National Wetlands Update August 2015 – Issue No 25, Commonwealth of Australia 2014’ The Commonwealth of Australia has made all reasonable efforts to identify content supplied by third parties using the following format ‘© Copyright, [name of third party] ’. Cover images Front cover: Wetlands provide important habitats for waterbirds, such as this adult great egret (Ardea modesta) at Leichhardt Lagoon in Queensland (© Copyright, Brian Furby) Back cover: Inland wetlands, like Narran Lakes Nature Reserve Ramsar site in New South Wales, support high numbers of waterbird breeding and provide refuge for birds during droughts (© Copyright, Dragi Markovic) ii / Wetlands Australia August 2014 Contents Introduction to Wetlands Australia August -

Newly Established Breeding Sites of the Barnacle Goose Branta Leucopsis in North-Western Europe – an Overview of Breeding Habitats and Colony Development
244 N. FEIGE et al.: New established breeding sites of the Barnacle Goose in North-western Europe Newly established breeding sites of the Barnacle Goose Branta leucopsis in North-western Europe – an overview of breeding habitats and colony development Nicole Feige, Henk P. van der Jeugd, Alexandra J. van der Graaf, Kjell Larsson, Aivar Leito & Julia Stahl Feige, N., H. P. van der Jeugd, A. J. van der Graaf, K. Larsson, A. Leito, A. & J. Stahl 2008: Newly established breeding sites of the Barnacle Goose Branta leucopsis in North-western Europe – an overview of breeding habitats and colony development. Vogelwelt 129: 244–252. Traditional breeding grounds of the Russian Barnacle Goose population are at the Barents Sea in the Russian Arctic. During the last decades, the population increased and expanded the breeding area by establishing new breeding colonies at lower latitudes. Breeding numbers outside arctic Russia amounted to about 12,000 pairs in 2005. By means of a questionnaire, information about breeding habitat characteristics and colony size, colony growth and goose density were collected from breeding areas outside Russia. This paper gives an overview about the new breeding sites and their development in Finland, Estonia, Sweden, Denmark, Germany, The Netherlands and Belgium. Statistical analyses showed significant differences in habitat characteristics and population parameters between North Sea and Baltic breeding sites. Colonies at the North Sea are growing rapidly, whereas in Sweden the growth has levelled off in recent years.I n Estonia numbers are even decreasing. On the basis of their breeding site choice, the flyway population of BarnacleG eese traditionally breeding in the Russian Arctic can be divided into three sub-populations: the Barents Sea population, the Baltic population and the North Sea population. -

A Guide to the Birds of Barrow Island
A Guide to the Birds of Barrow Island Operated by Chevron Australia This document has been printed by a Sustainable Green Printer on stock that is certified carbon in joint venture with neutral and is Forestry Stewardship Council (FSC) mix certified, ensuring fibres are sourced from certified and well managed forests. The stock 55% recycled (30% pre consumer, 25% post- Cert no. L2/0011.2010 consumer) and has an ISO 14001 Environmental Certification. ISBN 978-0-9871120-1-9 Gorgon Project Osaka Gas | Tokyo Gas | Chubu Electric Power Chevron’s Policy on Working in Sensitive Areas Protecting the safety and health of people and the environment is a Chevron core value. About the Authors Therefore, we: • Strive to design our facilities and conduct our operations to avoid adverse impacts to human health and to operate in an environmentally sound, reliable and Dr Dorian Moro efficient manner. • Conduct our operations responsibly in all areas, including environments with sensitive Dorian Moro works for Chevron Australia as the Terrestrial Ecologist biological characteristics. in the Australasia Strategic Business Unit. His Bachelor of Science Chevron strives to avoid or reduce significant risks and impacts our projects and (Hons) studies at La Trobe University (Victoria), focused on small operations may pose to sensitive species, habitats and ecosystems. This means that we: mammal communities in coastal areas of Victoria. His PhD (University • Integrate biodiversity into our business decision-making and management through our of Western Australia) -

Review of the Ecology, Status and Modelling of Waterbird Populations of the Coorong South Lagoon
Review of the ecology, status and modelling of waterbird populations of the Coorong South Lagoon Thomas A. A. Prowse Goyder Institute for Water Research Technical Report Series No. 20/12 www.goyderinstitute.org Goyder Institute for Water Research Technical Report Series ISSN: 1839-2725 The Goyder Institute for Water Research is a research alliance between the South Australian Government through the Department for Environment and Water, CSIRO, Flinders University, the University of Adelaide and the University of South Australia. The Institute facilitates governments, industries, and leading researchers to collaboratively identify, develop and adopt innovative solutions for complex water management challenges to ensure a sustainable future. This program is part of the Department for Environment and Water’s Healthy Coorong Healthy Basin Program, which is jointly funded by the Australian and South Australian Governments. Enquires should be addressed to: Goyder Institute for Water Research 209A Darling Building, North Terrace The University of Adelaide, Adelaide SA 5005 tel: (08) 8313 5020 e-mail: [email protected] Citation Prowse TAA (2020) Review of the ecology, status and modelling of waterbird populations of the Coorong South Lagoon. Goyder Institute for Water Research Technical Report Series No. 20/12. © Crown in right of the State of South Australia, Department for Environment and Water, The University of Adelaide. Disclaimer This report has been prepared by The University of Adelaide (as the Goyder Institute for Water Research partner organisation) and contains independent scientific/technical advice to inform government decision- making. The independent findings and recommendations of this report are subject to separate and further consideration and decision-making processes and do not necessarily represent the views of the Australian Government or the South Australian Department for Environment and Water. -
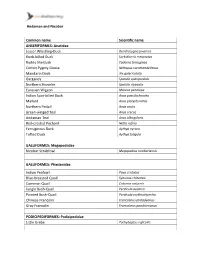
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

Bird Vulnerability Assessments
Assessing the vulnerability of native vertebrate fauna under climate change, to inform wetland and floodplain management of the River Murray in South Australia: Bird Vulnerability Assessments Attachment (2) to the Final Report June 2011 Citation: Gonzalez, D., Scott, A. & Miles, M. (2011) Bird vulnerability assessments- Attachment (2) to ‘Assessing the vulnerability of native vertebrate fauna under climate change to inform wetland and floodplain management of the River Murray in South Australia’. Report prepared for the South Australian Murray-Darling Basin Natural Resources Management Board. For further information please contact: Department of Environment and Natural Resources Phone Information Line (08) 8204 1910, or see SA White Pages for your local Department of Environment and Natural Resources office. Online information available at: http://www.environment.sa.gov.au Permissive Licence © State of South Australia through the Department of Environment and Natural Resources. You may copy, distribute, display, download and otherwise freely deal with this publication for any purpose subject to the conditions that you (1) attribute the Department as the copyright owner of this publication and that (2) you obtain the prior written consent of the Department of Environment and Natural Resources if you wish to modify the work or offer the publication for sale or otherwise use it or any part of it for a commercial purpose. Written requests for permission should be addressed to: Design and Production Manager Department of Environment and Natural Resources GPO Box 1047 Adelaide SA 5001 Disclaimer While reasonable efforts have been made to ensure the contents of this publication are factually correct, the Department of Environment and Natural Resources makes no representations and accepts no responsibility for the accuracy, completeness or fitness for any particular purpose of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of or reliance on the contents of this publication. -

A Year on the Vasse-Wonnerup Wetlands
Department of Biodiversity, Conservation and Attractions Department of Primary Industries and Regional Development Department of Water and Environmental Regulation A Year on the Vasse-Wonnerup Wetlands An Ecological Snapshot March 2017—January 2018 W IN N T M E U R T U A S P R R I N E G M M U S Musk duck (Biziura lobata) (Photo: Mark Oliver) The Vasse-Wonnerup Wetlands The conservation values of the Vasse-Wonnerup wetlands are recognised on a local, state, national and international level. The wetlands provide habitat to thousands of Australian and migratory water birds as well as supporting the largest breeding population of black swans in the state. In 1990 the wetlands were recognised as a ‘Wetland of International Importance’ under the Ramsar Convention. The wetlands are also one of the most nutrient enriched wetlands in Western Australia, characterised by extensive macroalgae and phytoplankton blooms and occasional major fish kills. Poor water quality in the wetlands is a major concern for the local community. Over the past four years scientists have been working together to investigate options to improve water quality in the wetlands by monitoring seawater inflows through the Vasse surge barrier and modelling options to increase water levels and flows into the Vasse estuary. These trials have successfully shown that seawater inflows can reduce the incidence of harmful phytoplankton blooms and improve conditions for fish in the Vasse Estuary Channel over summer months. What isn’t as well understood is how these management approaches and subsequent increased water levels may impact on the broader wetland system, especially how the ecology of the wetlands responds to changes in the timing and volume of seawater inflow through the surge barriers. -

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

Three Species of Siberian Geese Seen in Nebraska Rick Wright Nebraska Ornithologists' Union
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nebraska Bird Review Nebraska Ornithologists' Union 3-1985 Three Species of Siberian Geese Seen in Nebraska Rick Wright Nebraska Ornithologists' Union Alan Grenon Nebraska Ornithologists' Union Follow this and additional works at: http://digitalcommons.unl.edu/nebbirdrev Part of the Ornithology Commons, Poultry or Avian Science Commons, and the Zoology Commons Wright, Rick and Grenon, Alan, "Three Species of Siberian Geese Seen in Nebraska" (1985). Nebraska Bird Review. 937. http://digitalcommons.unl.edu/nebbirdrev/937 This Article is brought to you for free and open access by the Nebraska Ornithologists' Union at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Nebraska Bird Review by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Wright, Grenon & Rose, "Three Species of Siberian Geese Seen in Nebraska," from Nebraska Bird Review (March 1985) 53(1). Copyright 1985, Nebraska Ornithologists' Union. Used by permission. Nebraska Bird Review 3 THREE SPECIES OF' SIBERIAN GEESE SEEN IN NEBRASKA At about 3:00 PM on 29 December 1984, whi~e participating in the DeSoto NWR Christmas Count, Betty Grenon, David Starr, and the authors, Rick Wright and AIan Grenon, flushed from near the west shore of the DeSoto Cut-off (Washington Co., Nebraska) a party of seven Greater White-fronted Geese. With these seven geese was one distinctly larger, which drew our attention as the small flock flew above us for about five minutes. The larger bird displayed obvious damage to or loss of primaries on each wing, making it easier for the four of us to concentrate our observations on it and compare our impressions.