Patent Ductus Arteriosus (PDA)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patent Ductus Arteriosus
Peer Reviewed Correction of a Canine Left-to-Right Shunting By Connie K. Varnhagen, PhD, RAHT Patent Ductus Arteriosus Scrappy, a 5-year-old, 27-kg, neutered Labrador retriever, presented to the Calgary Animal Referral and Emergency (CARE) Centre in Calgary, Alberta, Canada, for mild exercise intolerance. The owner reported that the normally active dog was “slowing down” and pant- ing excessively even after mild exercise. History time and mucous membrane color were normal. Lung sounds The patient was the runt of its litter, was rejected by its were normal on auscultation. Based on the physical examina- dam, and was hand-fed for the first 5 days. A presumed tion, the differential diagnosis included patent ductus arterio- innocent heart murmur was detected on auscultation at the sus (PDA) or another type of arteriovenous fistula. puppy’s first veterinary examination. Right lateral and ventrodorsal (Figure 1) thoracic radio- At approximately 6 months of age, the patient began graphs revealed cardiomegaly with left atrial and ventricu- experiencing chronic small intestine diarrhea and weight lar enlargement and a prominent pulmonary artery bulge. loss. Over the next 30 months, the dog was diagnosed and Electrocardiography (ECG; Figure 2) demonstrated a tall treated for giardiasis, coccidiosis, small intestinal bacterial R wave (greater than 4.0 mV in lead II compared with a overgrowth, borderline exocrine pancreatic insufficiency, normal parameter of 3.0 mV), indicative of left ventricular and lymphocytic plasmacytic inflammatory bowel disease. enlargement, and normal sinus rhythm. Also at approximately 6 months of age, the patient Echocardiography was performed and was definitive developed a nonseasonal pruritus and dry, brittle haircoat. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -
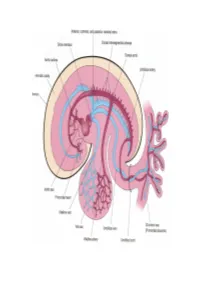
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Echocardiography of the Patent Ductus Arteriosus in Premature Infant
Received: 15 August 2018 | Accepted: 16 October 2018 DOI: 10.1111/chd.12703 SPECIAL ISSUE ARTICLE Echocardiography of the patent ductus arteriosus in premature infant Govinda Paudel MD | Vijaya Joshi MD Pediatric Cardiology, University of Tennessee Health Science Center, Le Abstract Bonheur Children’s Hospital, Memphis, Management of the patent ductus arteriosus (PDA) in the premature infant has been Tennessee a point of controversy for decades as smaller and earlier gestational age infants have Correspondence been surviving. Increasing experience with catheter‐based device closure has gener‐ Govinda Paudel, MD, Pediatric Cardiology, University of Tennessee Health Science ated a new wave of interest in this subject. In this era, echocardiography plays a cen‐ Center, Le Bonheur Children’s Hospital, 49 tral role for collaboration within a multispecialty team. Reliability of echocardiography North Dunlap Street, Memphis, TN, 38103. Email: [email protected] is improved by applying an institutionally derived standard approach to imaging, data collection, and reporting. The key aspects of both the physiology and anatomy of the PDA to distinguish infants that may benefit from intervention are described. KEYWORDS device closure, echocardiography, patent ductus arteriosus, premature infants 1 | INTRODUCTION cardiologists (including interventionist) and neonatologists should agree on the imaging parameters desired and reported. The report Patent ductus arteriosus (PDA) is associated with numerous com‐ summary should have a standard format that tracks serial changes plications in premature babies.1 Hemodynamics of the PDA guide from the previous echo as this is essential to optimize communication. management. But anatomic features have gained importance with Although high‐frequency probes (8‐12 MHz) are most commonly the growing experience in transcatheter device closure of the ductus utilized, lower frequency probes can provide better penetration from in premature infants. -

The Patent Ductus Arteriosus (PDA) and the Preterm Baby
12/5/2018 The Patent Ductus Arteriosus (PDA) and the Preterm Baby Tanya Hatfield, RNC - NIC, MSN Neonatal Outreach Educator Objectives ▪ Describe normal cardiac physiology and development ▪ Understand the unique physiologic needs of the preterm infant with a PDA ▪ Define the implications prematurity presents with the cardiac system 2 1 12/5/2018 Normal Cardiovascular Function: Review Uptodate.com,. (2015). Identifying newborns with critical congenital heart disease. Retrieved 22 October 2015, 3 from http://www.uptodate.com/contents/identifying - newborns - with - critical - congenital - heart - disease?source=search_result&search=congenital+heart+disease&selectedTitle=1~150 Normal Cardiovascular Function: Review Right Left Lungs Body Pulmonary Systemic Artery (PA) Aorta ( Ao ) Uptodate.com,. (2015). Identifying newborns with critical congenital heart disease. Retrieved 22 October 2015, 4 from http://www.uptodate.com/contents/identifying - newborns - with - critical - congenital - heart - disease?source=search_result&search=congenital+heart+disease&selectedTitle=1~150 2 12/5/2018 Fetal Circulation (2015). Retrieved 29 October 2015, from 5 http://higheredbcs.wiley.com/legacy/college/tortora/0470565101/hearthis_ill/pap13e_ch21_illustr_audio_m p3_am/simulations/hear/fetal_circulation.html Fetal Circulation ▪ Gas exchange is liquid to liquid ▪ Organ of respiration is placenta ▪ High flow, low resistance ▪ Fetal lungs • Low flow, high resistance ▪ PA’s constricted ▪ High right heart and lung pressures ▪ Low left heart pressures ▪ Open fetal -

Artery Umbilical Cord
THE PRACTICAL IMPORTANCE OF THE SINGLE ARTERY UMBILICAL CORD RICHARD F. HNAT Department of Obstetrics and Gynecology, The New York Hospital-Cornell Medical Center, New Yoi [Received 23rd May 1966) Summary. The correlation of single artery umbilical cords with con- genital anomalies is explored in the present study of 4808 consecutive deliveries. There were thirty-eight single artery cords, and six of these were associated with additional foetal abnormalities. An attempt is made to present the correlation in a true perspective by adding these data to other similar studies. Acknowledgment is given to the many retrospective studies, but consecutive delivery series show that a single artery cord is of importance as an abnormal physical finding but not as a screening test to detect occult malformations. INTRODUCTION Recent studies from large obstetrical services have suggested a correlation between the absence of one umbilical artery and congenital anomalies. Although this association had been mentioned before and after the observa¬ tion attributed to Hyrtl, it attracted little attention until the work of Ben- irschke & Brown (1955), a retrospective study of fifty-five placentae and autopsies of newborns from three different institutions. Of these infants, twenty-seven exhibited malformations involving all organ systems. Since 1955 many reports have appeared. Retrospective studies have taken the form of either autopsy material or accumulated cases, usually from different hospitals, added to the experience of the large teaching institution from which the report emanated (Table 1). Prospective studies consist entirely of cord specimens collected from consecutive deliveries, and they have consequently suffered from the small number of cases (Table 2). -

Patent Ductus Arteriosus with Pulmonary Hypertension by John A
Br Heart J: first published as 10.1136/hrt.15.4.423 on 1 October 1953. Downloaded from PATENT DUCTUS ARTERIOSUS WITH PULMONARY HYPERTENSION BY JOHN A. COSH From the Departnent ofMedicine, University of Bristol Received July 22, 1953 In most cases of patent ductus arteriosus the diagnosis is readily made on recognition of the typical murmur. In some, however, the continuous murmur is lacking, and a systolic murmur only, of variable loudness, is heard at the pulmonary area. This is not uncommon in infants and young children, who may later develop the characteristic murmur (Gilchrist, 1945). Occasionally the continuous murmur is absent in adults in whom patency of the ductus is found subsequently at autopsy: in such cases the state of the pulmonary arteries and the presence of right ventricular hypertrophy may indicate that the pulmonary arterial pressure was much raised in life (Holman, 1925; Keys and Shapiro, 1943 (Case 3); Chapman, 1944; Douglas et al., 1947; Ulrich, 1947). As a result, the pressure gradient between the aorta and the pulmonary artery may be so diminished that the blood flow along the ductus is insufficient to set up a continuous murmur. In extreme examples the pressure in the pulmonary artery may exceed that in the aorta, causing a reversal of the usual left to right shunt (Johnson et al., 1950; Campbell and Hudson, 1951; Dammann et al., 1953). Three cases of patent ductus arteriosus complicated by severe pulmonary hypertension are presented here. The diagnosis was made in each on cardiac catheterization. In two, pulmonary hypertension caused reversal of the shunt; in the third the shunt was not reversed, but there was, in addition, coarctation of the aorta. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery
Pediat. Res. 6: 693-700 (1972) Acetylcholine neonate atropine prematurity bradykinin sympathetic nervous system ductus arteriosus The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery INGRID OBERHANSLI-WEISS, MICHAEL A. HEYMANN1391, ABRAHAM M. RUDOLPH, AND KENNETH L. MELMON Cardiovascular Research Institute and Departments of Pediatrics, Physiology and Pharmacology, University of California San Francisco, San Francisco, California, USA Extract Response of the ductus arteriosus and umbilical artery to changes in oxygen tension, to acetylcholine, and to sympathetic and parasympathetic blocking agents was studied in vitro in isolated rings obtained from 22 fetal lambs of 98- to 147-day gestation. After stabilization of tension at a baseline level (0.3-0.7 g) in a PO2 environment of 35-45 mm Hg, both increase of the PO2 to 550 mm Hg and decrease of the PO2 to 8 mm Hg of the bathing solution produced constriction. The mean maximal tension developed by the ductus arteriosus.was 3.91 g at high PO2 and 3.87 g at low POr The increase in maximal tension developed with advancing gestation was also similar at both high and low POj. At P02 levels of 8-550 mm Hg, acetylcholine produced a further increase in tension, whereas bradykinin only produced an increase in tension at high PO2- Alpha and beta sympathetic blockade had no effect on the constrictor response to oxygen. Atropine relaxed the ductus arteriosus and umbilical artery at both high and low Po2 levels; the degree of relaxation was related to drug concentration. Acetylcholin- esterase also relaxed the ductus arteriosus constricted by oxygen. -

Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure
Acta Cardiol Sin 2016;32:731-737 Brief Report doi: 10.6515/ACS20160205A Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure Ali Yildirim,1 Alperen Aydin,2 Tevfik Demir,1 Fatma Aydin,2 Birsen Ucar1 and Zubeyir Kilic1 Background: The aim of the present study was to evaluate the echocardiographic follow-up of patent foramen ovale, which is considered a potential etiological factor in various diseases, and to determine the factors affecting spontaneous closure. Methods: Between January 2000 and June 2012, records of 918 patients with patent foramen ovale were retrospectively reviewed. Patency of less than 3 mm around the fossa ovalis is called patent foramen ovale. Patients with cyanotic congenital heart diseases, severe heart valve disorders and severe hemodynamic left to right shunts were excluded from the study. The patients were divided into three groups based on age; 1 day-1 monthingroup1,1month-12monthsingroup2,andmorethan12monthsingroup3. Results: Of the 918 patients, 564 (61.4%) had spontaneous closure, 328 (35.8%) had patent foramen ovale continued, 15 (1.6%) patients had patent foramen ovale enlarged to 3-5 mm, 6 patients were enlarged to 5-8 mm, and in one patient patent foramen ovale reached to more than 8 mm size. Defect was spontaneously closed in 65.9% of the patients in group 1, 66.7% of the patients in group 2, and 52.3% of the patients in group 3. There was a negative correlation between the age of diagnosis and spontaneous closure (p < 0.05). Gender, prematurity and coexisting malformations such as patent ductus arteriosus and atrial septal aneurysm did not have any effect on spontaneous closure of patent foramen ovale (p > 0.05). -

Concomitant Stenting of the Patent Ductus Arteriosus And
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Alwi et al Congenital Heart Disease Concomitant stenting of the patent ductus arteriosus and radiofrequency valvotomy in pulmonary atresia with intact ventricular septum and intermediate right ventricle: Early in-hospital and medium-term outcomes Mazeni Alwi, MRCP,a Kok-Kuan Choo, MRCP,a Nomee A. M. Radzi, MRCPCH,a Hasri Samion, MD,a Kiew-Kong Pau, FRCS,b and Chee-Chin Hew, FRCSb Objectives: Our objective was to determine the feasibility and early to medium-term outcome of stenting the CHD patent ductus arteriosus at the time of radiofrequency valvotomy in the subgroup of patients with pulmonary atresia with intact ventricular septum and intermediate right ventricle. Background: Stenting of the patent ductus arteriosus and radiofrequency valvotomy have been proposed as the initial intervention for patients with intermediate right ventricle inasmuch as the sustainability for biventricular circulation or 1½-ventricle repair is unclear in the early period. Methods: Between January 2001 and April 2009, of 143 patients with pulmonary atresia and intact ventricular septum, 37 who had bipartite right ventricle underwent radiofrequency valvotomy and stenting of the patent duc- tus arteriosus as the initial procedure. The mean tricuspid valve z-score wasÀ3.8 Æ 2.2 and the mean tricuspid valve/mitral valve ratio was 0.62 Æ 0.16. Results: Median age was 10 days (3–65 days) and median weight 3.1 kg (2.4–4.9 kg). There was no procedural mortality. Acute stent thrombosis developed in 1 patient and necessitated emergency systemic–pulmonary shunt. -

Pulmonary Atresia: Intact Ventricular Septum
© 2012 The Children’s Heart Clinic Normal Heart NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia with Intact Ventricular Septum (PA/IVS) Pulmonary atresia with intact ventricular septum (PA/IVS) refers to the absence or underdevelopment of the pulmonary valve and the absence of a communication between the lower two chamber of the heart (ventricles). The pulmonary valve ring and main pulmonary artery are hypoplastic (underdeveloped) due to lack of blood flow in utero. This means there is no direct communication between the right ventricle and the pulmonary artery. The only source of blood to the lungs is supplied by a patent ductus arteriosus (PDA), a normal fetal structure that usually closes in the first week of life. PA/IVS is often associated with coronary anomalies, such as obstruction or absence of the proximal or left coronary artery. The right ventricle (RV) size varies and portions of the right ventricle may be absent. The muscle (myocardium) of the right ventricle (RV) is often abnormal. PA/IVS accounts for less than 1% of all congenital heart defects and 2.5% of all critically ill infants with congenital heart disease. Physical Exam/Symptoms: Severe cyanosis (blue color) persists from birth. Tachypnea is often present.