Phosphorylation and Mutation of Phospholamban Alter Physical Interactions with the Sarcoplasmic Reticulum Calcium Pump
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pnas11138correction 14002..14003
Corrections MEDICAL SCIENCES Correction for “Regulation of bone remodeling by vasopressin New, Alberta Zallone, and Mone Zaidi, which appeared in issue 46, explains the bone loss in hyponatremia,” by Roberto Tamma, November 12, 2013, of Proc Natl Acad Sci USA (110:18644–18649; Li Sun, Concetta Cuscito, Ping Lu, Michelangelo Corcelli, first published October 28, 2013; 10.1073/pnas.1318257110). Jianhua Li, Graziana Colaianni, Surinder S. Moonga, Adriana The authors note that Fig. 1 appeared incorrectly. The cor- Di Benedetto, Maria Grano, Silvia Colucci, Tony Yuen, Maria I. rected figure and its legend appear below. Fig. 1. Bone cells express Avprs. Immunofluorescence micrographs (A) and Western immunoblotting (B) show the expression of Avpr1α in osteoblasts and osteoclasts, and as a function of osteoblast (mineralization) and osteoclast (with Rankl) differentiation. The expression of Avp (ligand) and Avpr1α (receptor) in osteoblasts is regulated by 17β-estradiol, as determined by quantitative PCR (C) and Western immunoblotting (D). (Magnification: A,63×.) Because Avp is a small peptide, its precursor neurophysin II is measured. Statistics: Student t test, P values shown compared with 0 h. Stimulation of Erk phosphorylation − (p-Erk) as a function of total Erk (t-Erk) by Avp (10 8 M) in osteoclast precursors (preosteoclasts), osteoclasts (OC), and osteoblasts establishes functionality of − the Avpr1α in the presence or absence of the receptor inhibitor SR49059 (10 8 M) (E). Western immunoblotting showing the expression of Avpr2 in pre- −/− osteoclasts, OCs (F), and osteoblasts (G) isolated from Avpr1α mice, as well as in MC3T3.E1 osteoblast precursors (G). Functionality of Avpr2 was confirmed −/− by the demonstration that cells from Avpr1α mice remained responsive to AVP in reducing the expression of osteoblast differentiation genes, namely Runx2, Osx, Bsp, Atf4, Opn, and Osteocalcin (quantitative PCR, P values shown) (H). -

Sat-196 How to Estimate Glomerular Filtration Rate
ISN WCN 2019 ABSTRACTS 13 (28%) patients had CKD stage 3. 6/13 had low predicted risk of Nephrology, Melbourne, Australia, 8Austin Hospital, Nephrology, Heidel- berg, Australia, 9Monash Childrens Hospital, Nephrology, Australia, progression at 5 years of whom 4/6 progressed unexpectedly. 1/13 was 10 identified as having high risk at 5 years and that 1 patient progressed to Melbourne Genomics Health Alliance, Victorian Clinical Genetics Service, Melbourne, Australia, 11Australian Genomic Health Alliance, KidGen Renal CKD5D/T. Genetics Flagship, Australia, Australia, 12Royal Childrens Hospital, 6/18 CKD 3/4 patients with predicted low risk progressed to CKD5D/ Nephrology, Melbourne, Australia T unexpectedly. 1/6 had emergency abdominal surgery, 1/6 patient had Introduction: Genomic technologies enable the rapid and cost-effective unexplained rapid progression and 4/6 had acute upper gastro-intes- fi tinal haemorrhage causing terminal decline of kidney function. sequencing of DNA and have demonstrated a de nitive diagnosis in Conclusions: The number of patients analysed was small. The 8-vari- several patient groups. The clinical utility of whole exome sequencing able equation accurately predicted high risk of progression to CKD5D/T (WES) in a kidney disease cohort is not yet well established. We in 7/9 CKD3/4 patients. describe the patient characteristics and diagnostic yield of a cohort of Conversely, 6/18 patients with predicted low risk progressed to 200 patients with suspected genetic kidney disease referred for WES via CKD5D/T. Acute medical events including upper gastro-intestinal bleed a multidisciplinary renal genetics clinic. Methods: accounted for most instances of unexpected progression. 200 sequential patients were recruited into a prospective observational cohort study through five tertiary academic centres in Victoria, Australia. -

Steroid-Dependent Regulation of the Oviduct: a Cross-Species Transcriptomal Analysis
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2015 Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis Katheryn L. Cerny University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cerny, Katheryn L., "Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis" (2015). Theses and Dissertations--Animal and Food Sciences. 49. https://uknowledge.uky.edu/animalsci_etds/49 This Doctoral Dissertation is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma
Research Article Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma Chunhua Lu,1 Tomas Bonome,3 Yang Li,1 Aparna A. Kamat,1 Liz Y. Han,1 Rosemarie Schmandt,1 Robert L. Coleman,1 David M. Gershenson,1 Robert B. Jaffe,4 MichaelJ. Birrer, 3 and AnilK. Sood 1,2 Departments of 1Gynecologic Oncology and 2Cancer Biology, University of Texas M. D. Anderson Cancer Center, Houston, Texas; 3Cell and Cancer Biology Branch, National Cancer Institute, Bethesda, Maryland; and 4Center for Reproductive Sciences, University of California, San Francisco, San Francisco, California Abstract the promise of such approaches. However, the full spectrum of Therapeutic strategies based on antiangiogenic approaches differences in the tumor vasculature compared with its normal are beginning to show great promise in clinical studies. counterpart is not known. Identification of additional targets on However, full realization of these approaches requires tumor endothelium may allow opportunities for developing new identification of key differences in gene expression between therapeutic approaches to inhibit angiogenesis in a tumor-specific endothelial cells from tumors versus their normal counter- manner. parts. Here, we examined gene expression differences in Higher levels of proangiogenic cytokines and angiogenesis are purified endothelial cells from 10invasive epithelial ovarian associated with an increased risk of metastasis and poor prognosis cancers and 5 normal ovaries using Affymetrix U133 Plus in ovarian cancer (5, 6). To date, a small number of breast, colon, 2.0microarrays. More than 400differentially expressed genes and brain cancers have been analyzed for gene expression changes were identified in tumor-associated endothelial cells. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Calcium Cycling Proteins and Heart Failure: Mechanisms and Therapeutics
Calcium cycling proteins and heart failure: mechanisms and therapeutics Andrew R. Marks J Clin Invest. 2013;123(1):46-52. https://doi.org/10.1172/JCI62834. Review Series Ca2+-dependent signaling is highly regulated in cardiomyocytes and determines the force of cardiac muscle contraction. Ca2+ cycling refers to the release and reuptake of intracellular Ca2+ that drives muscle contraction and relaxation. In failing hearts, Ca2+ cycling is profoundly altered, resulting in impaired contractility and fatal cardiac arrhythmias. The key defects in Ca2+ cycling occur at the level of the sarcoplasmic reticulum (SR), a Ca2+ storage organelle in muscle. Defects in the regulation of Ca2+ cycling proteins including the ryanodine receptor 2, cardiac (RyR2)/Ca2+ release channel macromolecular complexes and the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a)/phospholamban complex contribute to heart failure. RyR2s are oxidized, nitrosylated, and PKA hyperphosphorylated, resulting in “leaky” channels in failing hearts. These leaky RyR2s contribute to depletion of Ca2+ from the SR, and the leaking Ca2+ depolarizes cardiomyocytes and triggers fatal arrhythmias. SERCA2a is downregulated and phospholamban is hypophosphorylated in failing hearts, resulting in impaired SR Ca2+ reuptake that conspires with leaky RyR2 to deplete SR Ca2+. Two new therapeutic strategies for heart failure (HF) are now being tested in clinical trials: (a) fixing the leak in RyR2 channels with a novel class of Ca2+-release channel stabilizers called Rycals and (b) increasing expression of SERCA2a to improve SR Ca2+ reuptake with viral-mediated gene therapy. There are many potential opportunities for additional mechanism-based therapeutics involving the machinery […] Find the latest version: https://jci.me/62834/pdf Review series Calcium cycling proteins and heart failure: mechanisms and therapeutics Andrew R. -
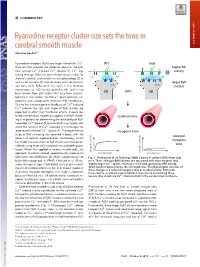
Ryanodine Receptor Cluster Size Sets the Tone in Cerebral Smooth Muscle
COMMENTARY Ryanodine receptor cluster size sets the tone in cerebral smooth muscle COMMENTARY Christian Soellera,1 + Ryanodine receptors (RyRs) are large intracellular Ca2 WT mdx channels that provide the molecular basis of the pro- K+ K+ higher BK + + + cess termed Ca2 -induced Ca2 release (1). Ca2 sig- activity naling through RyRs has been shown to be critical for CaC BK BK skeletal, cardiac, and smooth muscle physiology (2) as well as for neurons (3) and secretory cells like pancre- larger RyR atic beta cells. RyRs were first seen in the electron RyRs RyRs clusters microscope as ∼30-nm-size particles (4), and it had SMCs been known from EM studies that they form clusters, SR SR typically in the narrow “junctional” space between sar- colemma and sarcoplasmic reticulum (SR) membranes. + Due to the inherent positive feedback of Ca2 -induced + Ca2 release, the size and shape of RyR clusters are expected to affect their functional activity. Indeed, de- SMCs SMCs tailed mathematical modeling suggests that RyR cluster- cerebral artery ing is important for determining the excitability of RyR- + mediated Ca2 release (5, 6) and could, in principle, also + affect the amount of Ca2 released in microscopic re- 2+ lease events termed Ca sparks (7). The experimental myogenic tone study of RyR clustering has received a boost with the reduced advent of optical superresolution microscopy, which myogenic has made the assessment of RyR cluster size more acces- sible by using essentially standard immunolabeling pro- tone tocols. When first applied in cardiac muscle cells, this myogenic tone (%) tone myogenic approach revealed a broad, approximately exponential vessel pressure (%) tone myogenic vessel pressure RyR cluster size distribution (8), which had previously not Fig. -

For Imaging the Endoplasmic Reticulum Calcium Store
RESEARCH ARTICLE A Low Affinity GCaMP3 Variant (GCaMPer) for Imaging the Endoplasmic Reticulum Calcium Store Mark J. Henderson1*, Heather A. Baldwin1, Christopher A. Werley2, Stefano Boccardo2, Leslie R. Whitaker1, Xiaokang Yan1, Graham T. Holt6, Eric R. Schreiter6, Loren L. Looger6, Adam E. Cohen2,3,4,5, Douglas S. Kim6, Brandon K. Harvey1* 1 National Institute on Drug Abuse, National Institutes of Health, 251 Bayview Blvd, Baltimore, Maryland, 21224, United States of America, 2 Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts, 02138, United States of America, 3 Department of Physics, Harvard University, Cambridge, Massachusetts, 02138, United States of America, 4 Harvard Stem Cell Institute, Harvard University, Cambridge, Massachusetts, 02138, United States of America, 5 Howard Hughes Medical Institute, Harvard University, Cambridge, Massachusetts, 02138, United States of America, 6 Janelia Research Campus, Howard Hughes Medical Institute, 19700 Helix Drive, Ashburn, Virginia, 20147, United States of America * [email protected] (MJH); [email protected] (BKH) OPEN ACCESS Citation: Henderson MJ, Baldwin HA, Werley CA, Abstract Boccardo S, Whitaker LR, Yan X, et al. (2015) A Low Affinity GCaMP3 Variant (GCaMPer) for Imaging the Endoplasmic reticulum calcium homeostasis is critical for cellular functions and is disrupted in Endoplasmic Reticulum Calcium Store. PLoS ONE diverse pathologies including neurodegeneration and cardiovascular disease. Owing to the 10(10): e0139273. doi:10.1371/journal.pone.0139273 high concentration of calcium within the ER, studying this subcellular compartment requires Editor: Agustín Guerrero-Hernandez, Cinvestav-IPN, tools that are optimized for these conditions. To develop a single-fluorophore genetically MEXICO encoded calcium indicator for this organelle, we targeted a low affinity variant of GCaMP3 to Received: May 12, 2015 the ER lumen (GCaMPer (10.19)). -

Biophysical Interaction Between Phospholamban and Protein Phosphatase 1 Regulatory Subunit GM
View metadata,FEBS Letters citation 439 and (1998) similar 224^230 papers at core.ac.uk broughtFEBS to you 21118 by CORE provided by Elsevier - Publisher Connector Biophysical interaction between phospholamban and protein phosphatase 1 regulatory subunit GM Isabelle Berrebi-Bertrand*, Michel Souchet, Jean-Claude Camelin, Marie-Paule Laville, Thierry Calmels, Antoine Bril SmithKline Beecham Laboratoires Pharmaceutiques, 4 rue du Chesnay Beauregard, P.O. Box 58, 35762 Saint-Greègoire, France Received 29 September 1998 acid, can increase the activity of the calcium pump [2]. In a Abstract Regulation of the sarco(endo)plasmic reticulum Ca2+- ATPase (SERCA 2a) depends on the phosphorylation state of dephosphorylated form, PLB decreases the a¤nity of SERCA phospholamban (PLB). When PLB is phosphorylated, its 2a for calcium and phosphorylation of PLB removes its in- inhibitory effect towards SERCA 2a is relieved, leading to an hibitory e¡ect towards SERCA 2a [3] which is able to pump enhanced myocardial performance. This process is reversed by a more rapidly the Ca2 from the cytosol into the SR [4]; this sarcoplasmic reticulum (SR)-associated type 1 protein phospha- latter e¡ect is correlated with an enhanced relaxation of the tase (PP1) composed of a catalytic subunit PP1C and a heart [5]. Phosphorylation of PLB at distinct sites by cAMP- regulatory subunit GM. Human GM and PLB have been dependent (at serine 16) and calmodulin-dependent (at threo- produced in an in vitro transcription/translation system and used nine 17) protein kinases is reversed by a sarcoplasmic reticu- for co-immunoprecipitation and biosensor experiments. The lum (SR)-associated protein phosphatase. -

Structure and Function of the Human Ryanodine Receptors and Their Association with Myopathies—Present State, Challenges, and Perspectives
molecules Review Structure and Function of the Human Ryanodine Receptors and Their Association with Myopathies—Present State, Challenges, and Perspectives Vladena Bauerová-Hlinková * , Dominika Hajdúchová † and Jacob A. Bauer Institute of Molecular Biology, Slovak Academy of Sciences, Dúbravská Cesta 21, 845 51 Bratislava, Slovakia; [email protected] (D.H.); [email protected] (J.A.B.) * Correspondence: [email protected]; Tel.: +421-2-5930-7439 † Current address: Department of Pathological Physiology, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Malá Hora 4C, 036 01 Martin, Slovakia. Academic Editor: Jacopo Sgrignani and Giovanni Grazioso Received: 31 July 2020; Accepted: 30 August 2020; Published: 4 September 2020 Abstract: Cardiac arrhythmias are serious, life-threatening diseases associated with the dysregulation of Ca2+ influx into the cytoplasm of cardiomyocytes. This dysregulation often arises from dysfunction of ryanodine receptor 2 (RyR2), the principal Ca2+ release channel. Dysfunction of RyR1, the skeletal muscle isoform, also results in less severe, but also potentially life-threatening syndromes. The RYR2 and RYR1 genes have been found to harbor three main mutation “hot spots”, where mutations change the channel structure, its interdomain interface properties, its interactions with its binding partners, or its dynamics. In all cases, the result is a defective release of Ca2+ ions from the sarcoplasmic reticulum into the myocyte cytoplasm. Here, we provide an overview of the most frequent diseases resulting from mutations to RyR1 and RyR2, briefly review some of the recent experimental structural work on these two molecules, detail some of the computational work describing their dynamics, and summarize the known changes to the structure and function of these receptors with particular emphasis on their N-terminal, central, and channel domains. -

Mechanisms and Models of Cardiac Excitation-Contraction Coupling
Mechanisms and Models of Cardiac Excitation-Contraction Coupling R.L. Winslow1,R.Hinch2 and J.L. Greenstein1 1 Center for Cardiovascular Bioinformatics and Modeling and The Whitaker Biomedical Engineering Institute, The Johns Hopkins University School of Medicine and Whiting School of Engineering 2 Mathematical Institute, University of Oxford 1 Introduction Intracellular calcium (Ca2+) concentration plays an important regulatory role in a number of cellular processes. Cellular influx of Ca2+ activates intracel- lular signaling pathways that in turn regulate gene expression. Studies have identified over 300 genes and 30 transcription factors which are regulated by intracellular Ca2+ [1, 2]. Fluctuation of intracellular Ca2+ levels is also known to regulate intracellular metabolism by activation of mitochondrial matrix de- hydrogenases. The subsequent effects on the tri-carboxylic acid cycle increase the supply of reducing equivalents (NADH, FADH2), stimulating increased flux of electrons through the respiratory chain [3]. Most importantly, Ca2+ is a key signaling molecule in excitation-contraction (EC) coupling, the process by which electrical activation of the cell is coupled to mechanical contraction and force generation. The purpose of this chapter is to review the important role of Ca2+ in cardiac EC coupling, with a particular focus on presentation of quantitative models of the EC coupling process. One of the most remarkable features of cardiac EC coupling is that structural and molecular properties at the mi- croscopic scale have a profound influence on myocyte function at the macro- scopic scale. Thus, modeling of cardiac EC coupling is confronted with the enormous challenge of needing to integrate diverse information collected at multiple scales of biological analysis into comprehensive models.