Gene Alterations Identified by Expression Profiling in Tumor-Associated Endothelial Cells from Invasive Ovarian Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pnas11138correction 14002..14003
Corrections MEDICAL SCIENCES Correction for “Regulation of bone remodeling by vasopressin New, Alberta Zallone, and Mone Zaidi, which appeared in issue 46, explains the bone loss in hyponatremia,” by Roberto Tamma, November 12, 2013, of Proc Natl Acad Sci USA (110:18644–18649; Li Sun, Concetta Cuscito, Ping Lu, Michelangelo Corcelli, first published October 28, 2013; 10.1073/pnas.1318257110). Jianhua Li, Graziana Colaianni, Surinder S. Moonga, Adriana The authors note that Fig. 1 appeared incorrectly. The cor- Di Benedetto, Maria Grano, Silvia Colucci, Tony Yuen, Maria I. rected figure and its legend appear below. Fig. 1. Bone cells express Avprs. Immunofluorescence micrographs (A) and Western immunoblotting (B) show the expression of Avpr1α in osteoblasts and osteoclasts, and as a function of osteoblast (mineralization) and osteoclast (with Rankl) differentiation. The expression of Avp (ligand) and Avpr1α (receptor) in osteoblasts is regulated by 17β-estradiol, as determined by quantitative PCR (C) and Western immunoblotting (D). (Magnification: A,63×.) Because Avp is a small peptide, its precursor neurophysin II is measured. Statistics: Student t test, P values shown compared with 0 h. Stimulation of Erk phosphorylation − (p-Erk) as a function of total Erk (t-Erk) by Avp (10 8 M) in osteoclast precursors (preosteoclasts), osteoclasts (OC), and osteoblasts establishes functionality of − the Avpr1α in the presence or absence of the receptor inhibitor SR49059 (10 8 M) (E). Western immunoblotting showing the expression of Avpr2 in pre- −/− osteoclasts, OCs (F), and osteoblasts (G) isolated from Avpr1α mice, as well as in MC3T3.E1 osteoblast precursors (G). Functionality of Avpr2 was confirmed −/− by the demonstration that cells from Avpr1α mice remained responsive to AVP in reducing the expression of osteoblast differentiation genes, namely Runx2, Osx, Bsp, Atf4, Opn, and Osteocalcin (quantitative PCR, P values shown) (H). -

Sat-196 How to Estimate Glomerular Filtration Rate
ISN WCN 2019 ABSTRACTS 13 (28%) patients had CKD stage 3. 6/13 had low predicted risk of Nephrology, Melbourne, Australia, 8Austin Hospital, Nephrology, Heidel- berg, Australia, 9Monash Childrens Hospital, Nephrology, Australia, progression at 5 years of whom 4/6 progressed unexpectedly. 1/13 was 10 identified as having high risk at 5 years and that 1 patient progressed to Melbourne Genomics Health Alliance, Victorian Clinical Genetics Service, Melbourne, Australia, 11Australian Genomic Health Alliance, KidGen Renal CKD5D/T. Genetics Flagship, Australia, Australia, 12Royal Childrens Hospital, 6/18 CKD 3/4 patients with predicted low risk progressed to CKD5D/ Nephrology, Melbourne, Australia T unexpectedly. 1/6 had emergency abdominal surgery, 1/6 patient had Introduction: Genomic technologies enable the rapid and cost-effective unexplained rapid progression and 4/6 had acute upper gastro-intes- fi tinal haemorrhage causing terminal decline of kidney function. sequencing of DNA and have demonstrated a de nitive diagnosis in Conclusions: The number of patients analysed was small. The 8-vari- several patient groups. The clinical utility of whole exome sequencing able equation accurately predicted high risk of progression to CKD5D/T (WES) in a kidney disease cohort is not yet well established. We in 7/9 CKD3/4 patients. describe the patient characteristics and diagnostic yield of a cohort of Conversely, 6/18 patients with predicted low risk progressed to 200 patients with suspected genetic kidney disease referred for WES via CKD5D/T. Acute medical events including upper gastro-intestinal bleed a multidisciplinary renal genetics clinic. Methods: accounted for most instances of unexpected progression. 200 sequential patients were recruited into a prospective observational cohort study through five tertiary academic centres in Victoria, Australia. -

Supplemental Table S1
Entrez Gene Symbol Gene Name Affymetrix EST Glomchip SAGE Stanford Literature HPA confirmed Gene ID Profiling profiling Profiling Profiling array profiling confirmed 1 2 A2M alpha-2-macroglobulin 0 0 0 1 0 2 10347 ABCA7 ATP-binding cassette, sub-family A (ABC1), member 7 1 0 0 0 0 3 10350 ABCA9 ATP-binding cassette, sub-family A (ABC1), member 9 1 0 0 0 0 4 10057 ABCC5 ATP-binding cassette, sub-family C (CFTR/MRP), member 5 1 0 0 0 0 5 10060 ABCC9 ATP-binding cassette, sub-family C (CFTR/MRP), member 9 1 0 0 0 0 6 79575 ABHD8 abhydrolase domain containing 8 1 0 0 0 0 7 51225 ABI3 ABI gene family, member 3 1 0 1 0 0 8 29 ABR active BCR-related gene 1 0 0 0 0 9 25841 ABTB2 ankyrin repeat and BTB (POZ) domain containing 2 1 0 1 0 0 10 30 ACAA1 acetyl-Coenzyme A acyltransferase 1 (peroxisomal 3-oxoacyl-Coenzyme A thiol 0 1 0 0 0 11 43 ACHE acetylcholinesterase (Yt blood group) 1 0 0 0 0 12 58 ACTA1 actin, alpha 1, skeletal muscle 0 1 0 0 0 13 60 ACTB actin, beta 01000 1 14 71 ACTG1 actin, gamma 1 0 1 0 0 0 15 81 ACTN4 actinin, alpha 4 0 0 1 1 1 10700177 16 10096 ACTR3 ARP3 actin-related protein 3 homolog (yeast) 0 1 0 0 0 17 94 ACVRL1 activin A receptor type II-like 1 1 0 1 0 0 18 8038 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 1 0 0 0 0 19 8751 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 1 0 0 0 0 20 8728 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 1 0 0 0 0 21 81792 ADAMTS12 ADAM metallopeptidase with thrombospondin type 1 motif, 12 1 0 0 0 0 22 9507 ADAMTS4 ADAM metallopeptidase with thrombospondin type 1 -

Promotion of Periostin Expression Contributes to the Migration of Schwann Cells Eva Sonnenberg-Riethmacher1,2,*, Michaela Miehe2,3,* and Dieter Riethmacher1,2,‡
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3345-3355 doi:10.1242/jcs.174177 RESEARCH ARTICLE Promotion of periostin expression contributes to the migration of Schwann cells Eva Sonnenberg-Riethmacher1,2,*, Michaela Miehe2,3,* and Dieter Riethmacher1,2,‡ ABSTRACT Michailov et al., 2004; Riethmacher et al., 1997; Taveggia et al., Neuregulin ligands and their ErbB receptors are important for the 2005). Those experiments revealed that, later in development, development of Schwann cells, the glial cells of the peripheral mutant embryos exhibit a total loss of Schwann cells along their nervous system (PNS). ErbB3 deficiency is characterized by a peripheral axons, whereas pre-migratory Schwann cells are present complete loss of Schwann cells along axons of the peripheral nerves, near to the dorsal root ganglia but fail to migrate along the axons. impaired fasciculation and neuronal cell death. We performed The impaired glial cell migration in these mutants indicates a direct comparative gene expression analysis of dorsal root ganglia (DRG) function in migration. This interpretation is supported by the finding – explant cultures from ErbB3-deficient and wild-type mice in order to that mutations in the neuregulin ErbB signalling system lead to identify genes that are involved in Schwann cell development and changes in neural crest migration in mice (Britsch et al., 1998) and migration. The extracellular matrix (ECM) gene periostin was found to in directed Schwann cell migration in zebrafish (Lyons et al., 2005). exhibit the most prominent down regulation in ErbB3-deficient DRG. However, an earlier function in fate specification and/or Expression analysis revealed that the periostin-expressing cell proliferation that could in turn be a prerequisite for migration population in the PNS corresponds to Schwann cell precursors and cannot be completely ruled out. -

Comparison of Gene Expression Profiles in Chromate Transformed BEAS-2B Cells
Comparison of Gene Expression Profiles in Chromate Transformed BEAS-2B Cells Hong Sun1, Harriet A. Clancy1, Thomas Kluz1, Jiri Zavadil2, Max Costa1* 1 Nelson Institute of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, United States of America, 2 Department of Pathology, NYU Cancer Institute and Center for Health Informatics and Bioinformatics, NYU Langone Medical Center, New York, New York, United States of America Abstract Background: Hexavalent chromium [Cr(VI)] is a potent human carcinogen. Occupational exposure has been associated with increased risk of respiratory cancer. Multiple mechanisms have been shown to contribute to Cr(VI) induced carcinogenesis, including DNA damage, genomic instability, and epigenetic modulation, however, the molecular mechanism and downstream genes mediating chromium’s carcinogenicity remain to be elucidated. Methods/Results: We established chromate transformed cell lines by chronic exposure of normal human bronchial epithelial BEAS-2B cells to low doses of Cr(VI) followed by anchorage-independent growth. These transformed cell lines not only exhibited consistent morphological changes but also acquired altered and distinct gene expression patterns compared with normal BEAS-2B cells and control cell lines (untreated) that arose spontaneously in soft agar. Interestingly, the gene expression profiles of six Cr(VI) transformed cell lines were remarkably similar to each other yet differed significantly from that of either control cell lines or normal BEAS-2B cells. A total of 409 differentially expressed genes were identified in Cr(VI) transformed cells compared to control cells. Genes related to cell-to-cell junction were upregulated in all Cr(VI) transformed cells, while genes associated with the interaction between cells and their extracellular matrices were down-regulated. -

Steroid-Dependent Regulation of the Oviduct: a Cross-Species Transcriptomal Analysis
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2015 Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis Katheryn L. Cerny University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cerny, Katheryn L., "Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis" (2015). Theses and Dissertations--Animal and Food Sciences. 49. https://uknowledge.uky.edu/animalsci_etds/49 This Doctoral Dissertation is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -
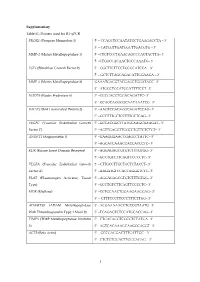
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Tepzz¥ 6Z54za T
(19) TZZ¥ ZZ_T (11) EP 3 260 540 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 27.12.2017 Bulletin 2017/52 C12N 15/113 (2010.01) A61K 9/127 (2006.01) A61K 31/713 (2006.01) C12Q 1/68 (2006.01) (21) Application number: 17000579.7 (22) Date of filing: 12.11.2011 (84) Designated Contracting States: • Sarma, Kavitha AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Philadelphia, PA 19146 (US) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Borowsky, Mark PL PT RO RS SE SI SK SM TR Needham, MA 02494 (US) • Ohsumi, Toshiro Kendrick (30) Priority: 12.11.2010 US 412862 P Cambridge, MA 02141 (US) 20.12.2010 US 201061425174 P 28.07.2011 US 201161512754 P (74) Representative: Clegg, Richard Ian et al Mewburn Ellis LLP (62) Document number(s) of the earlier application(s) in City Tower accordance with Art. 76 EPC: 40 Basinghall Street 11840099.3 / 2 638 163 London EC2V 5DE (GB) (71) Applicant: The General Hospital Corporation Remarks: Boston, MA 02114 (US) •Thecomplete document including Reference Tables and the Sequence Listing can be downloaded from (72) Inventors: the EPO website • Lee, Jeannie T •This application was filed on 05-04-2017 as a Boston, MA 02114 (US) divisional application to the application mentioned • Zhao, Jing under INID code 62. San Diego, CA 92122 (US) •Claims filed after the date of receipt of the divisional application (Rule 68(4) EPC). (54) POLYCOMB-ASSOCIATED NON-CODING RNAS (57) This invention relates to long non-coding RNAs (IncRNAs), libraries of those ncRNAs that bind chromatin modifiers, such as Polycomb Repressive Complex 2, inhibitory nucleic acids and methods and compositions for targeting IncRNAs. -

Periostin Modulates Myofibroblast Differentiation During Full-Thickness Cutaneous Wound Repair
Research Article 121 Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair Christopher G. Elliott1, Jian Wang2, Xiaolei Guo3, Shi-wen Xu4, Mark Eastwood5, Jianjun Guan3, Andrew Leask6, Simon J. Conway2 and Douglas W. Hamilton1,6,* 1Department of Anatomy and Cell Biology, Schulich School of Medicine and Dentistry, The University of Western Ontario, 1151 Richmond St, London, Ontario, Canada, N6A 5C1 2Riley Heart Research Center, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 West Walnut, Indianapolis, IN 46202, USA 3Department of Materials Science and Engineering, The Ohio State University, 2041 College Road, Columbus, OH 43210, USA 4Centre for Rheumatology, Royal Free and University College Medical School, London, NW3 2PF, UK 5School of Life Sciences, University of Westminster, London, W1B 2UW, UK 6Division of Oral Biology, Schulich School of Medicine and Dentistry, The University of Western Ontario, 1151 Richmond St, London, Ontario, Canada, N6A 5C1 *Author for correspondence ([email protected]) Accepted 22 July 2011 Journal of Cell Science 125, 121–132 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.087841 Summary The matricellular protein periostin is expressed in the skin. Although periostin has been hypothesized to contribute to dermal homeostasis and repair, this has not been directly tested. To assess the contribution of periostin to dermal healing, 6 mm full-thickness excisional wounds were created in the skin of periostin-knockout and wild-type, sex-matched control mice. In wild-type mice, periostin was potently induced 5–7 days after wounding. In the absence of periostin, day 7 wounds showed a significant reduction in myofibroblasts, as visualized by expression of a-smooth muscle actin (a-SMA) within the granulation tissue. -

Characterization of a 7.6-Mb Germline Deletion Encompassing the NF1 Locus and About a Hundred Genes in an NF1 Contiguous Gene Syndrome Patient
European Journal of Human Genetics (2008) 16, 1459–1466 & 2008 Macmillan Publishers Limited All rights reserved 1018-4813/08 $32.00 www.nature.com/ejhg ARTICLE Characterization of a 7.6-Mb germline deletion encompassing the NF1 locus and about a hundred genes in an NF1 contiguous gene syndrome patient Eric Pasmant*,1,2, Aure´lie de Saint-Trivier2, Ingrid Laurendeau1, Anne Dieux-Coeslier3, Be´atrice Parfait1,2, Michel Vidaud1,2, Dominique Vidaud1,2 and Ivan Bie`che1,2 1UMR745 INSERM, Universite´ Paris Descartes, Faculte´ des Sciences Pharmaceutiques et Biologiques, Paris, France; 2Service de Biochimie et de Ge´ne´tique Mole´culaire, Hoˆpital Beaujon AP-HP, Clichy, France; 3Service de Ge´ne´tique Clinique, Hoˆpital Jeanne de Flandre, Lille, France We describe a large germline deletion removing the NF1 locus, identified by heterozygosity mapping based on microsatellite markers, in an 8-year-old French girl with a particularly severe NF1 contiguous gene syndrome. We used gene-dose mapping with sequence-tagged site real-time PCR to locate the deletion end points, which were precisely characterized by means of long-range PCR and nucleotide sequencing. The deletion is located on chromosome arm 17q and is exactly 7 586 986 bp long. It encompasses the entire NF1 locus and about 100 other genes, including numerous chemokine genes, an attractive in silico-selected cerebrally expressed candidate gene (designated NUFIP2, for nuclear fragile X mental retardation protein interacting protein 2; NM_020772) and four microRNA genes. Interestingly, the centromeric breakpoint is located in intron 4 of the PIPOX gene (pipecolic acid oxidase; NM_016518) and the telomeric breakpoint in intron 5 of the GGNBP2 gene (gametogenetin binding protein 2; NM_024835) coding a transcription factor. -

Targeting PH Domain Proteins for Cancer Therapy
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 12-2018 Targeting PH domain proteins for cancer therapy Zhi Tan Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Bioinformatics Commons, Medicinal Chemistry and Pharmaceutics Commons, Neoplasms Commons, and the Pharmacology Commons Recommended Citation Tan, Zhi, "Targeting PH domain proteins for cancer therapy" (2018). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 910. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/910 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. TARGETING PH DOMAIN PROTEINS FOR CANCER THERAPY by Zhi Tan Approval page APPROVED: _____________________________________________ Advisory Professor, Shuxing Zhang, Ph.D. _____________________________________________ -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase