United Nations Environment Programme Cep Technical Report 2007
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
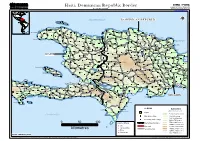
Haiti, Dominican Republic Border Geographic Information and Mapping Unit As of February 2004 Population and Geographic Data Section Email : [email protected]
GIMU / PGDS Haiti, Dominican Republic Border Geographic Information and Mapping Unit As of February 2004 Population and Geographic Data Section Email : [email protected] ATLANTIC OCEAN DOMINICANDOMINICAN REPUBLICREPUBLIC !!! Voute I Eglise ))) ))) Fond Goriose ))) ))) ))) Saint Louis du Nord ))) ))) ))) ))) Cambronal Almaçenes ))) ))) ))) ))) ))) Monte Cristi ))) Jean Rabel ))) ))) Bajo Hondo ))) ))) ))) Gélin ))) ))) ))) ))) ))) Sabana Cruz ))) La Cueva ))) Beau Champ ))) ))) Haiti_DominicanRepBorder_A3LC Mole-Saint-Nicolas ))) ))) ))) ))) ))) Bassin ))) Barque ))) Los Icacos ))) ))) Bajo de Gran Diablo )))Puerto Plata ))) Bellevue ))) Beaumond CAPCAPCAP HAITIEN HAITIENHAITIEN ))) Palo Verde CAPCAPCAP HAITIEN )HAITIEN)HAITIEN) ))) PUERTOPUERTOPUERTO PLATA PLATAPLATA INTERNATIONAL INTERNATIONALINTERNATIONAL ))) ))) Bambou ))) ))) Imbert ))) VVPUERTOPUERTOPUERTO))) PLATA PLATAPLATA INTERNATIONAL INTERNATIONALINTERNATIONAL VV ))) VV ))) ))) ))) VV ))) Sosúa ))) ))) ))) Atrelle Limbé VV ))) ))) ))) ))) VV ))) ))) ))) ))) VV ))) ))) ))) Fatgunt ))) Chapereau VV Lucas Evangelista de Peña ))) Agua Larga ))) El Gallo Abajo ))) ))) ))) ))) Grande Plaine Pepillo Salcedo))) ))) Baitoa ))) ))) ))) Ballon ))) ))) ))) Cros Morne))) ))) ))) ))) ))) Sabaneta de Yásica ))) Abreu ))) ))) Ancelin ))) Béliard ))) ))) Arroyo de Leche Baie-de-Henne ))) ))) Cañucal ))) ))) ))) ))) ))) La Plateforme ))) Sources))) Chaudes ))) ))) Terrier Rouge))) Cacique Enriquillo ))) Batey Cerro Gordo ))) Aguacate del Limón ))) Jamao al Norte ))) ))) ))) Magante Terre Neuve -

United States National Museum
SMITHSONIAN INSTITUTION UNITED STATES NATIONAL MUSEUM Bulletin 156 ABORIGINAL INDIAN POTTERY OF THE DOMINICAN REPUBLIC BY HERBERT W. KRIEGER Curator of Ethnology, United States National Museum UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1931 For sale by the Superintendent of Documents, Washington, D. C. Price, 75 cents ADVERTISEMENT The scientific publications of the National Museum include two series, known, respectively, as Proceedings and Bulletins. The Proceedings^ begun in 1878, are intended primarily as a me- dium for the publication of original papers, based on the collections of the National Museum, that set forth newly acquired facts in biology, anthropology, and geology, with descriptions of new forms and revisions of limited groups. Copies of each paper, in pamphlet form, are distributed as published to libraries and scientific organiza- tions and to specialists and others interested in the different subjects. The dates at which these separate papers are published are recorded in the table of contents of each of the volumes. The Bulletins^ the first of which was issued in 1875, consist of a series of separate publications comprising monographs of large zoological groups and other general sj^stematic treatises (occasion- ally in several volumes), faunal works, reports of expeditions, cata- logues of type-specimens, special collections, and other material of a similar nature. The majority of the volumes are octavo in size, but a quarto size has been adopted in a few instances in which large plates were regarded as indispensable. In the Bulletin series appear volumes under the heading Gontrihutions from the United States National Hei'haHum^ in octavo form, published by the National Museum since 1902, which contain papers relating to the botanical collections of the Museum. -

Reptilla: Sauria: Iguanidae
~moo~~~~oo~~moo~ ~oorn~m~m~~ rnoo~rnrnm~m~~ Number 47 December 30, 1981 Variation in Hispaniolan Anolis olssoni Schmidt (Reptilia: Sauria: Iguanidae) Albert Schwartz REVIEW COMMITTEE FOR THIS PUBLICATION: Ronald Crombie, National Museum of Natural History Robert Henderson, Milwaukee Public Museum Richard Thomas, University of Puerto Rico ISBN 0·89326·077·0 Accepted for publication November 11, 1981 Milwaukee Public Museum Publications Published by the order of the Board of Trustees Milwaukee Public Museum 800 West Wells Street Milwaukee, Wisconsin 53233 Variation in Hispaniolan Anolis olssoni Schmidt (Reptilia: Sauria: Iguanidae) Albert Schwartz Miami-Dade Community College North Campus, Miami, Florida 33167 ABSTRACT - The Hispaniolan "grass anole," Anolis olssoni Schmidt, is widespread on the Hispaniolan north island and has invaded the south island Peninsula de Barahona and De de la Gonave. Although these lizards are xerophilic, their dorsal color and pattern are not always pale as is typical of desert lizards. Seven subspecies are recognized on the main island and another on De de la Gonave. These differ in color, pattern, and scutellation, and at least two are apparently widely disjunct from other populations. Two Dominican and two Haitian populations are left unassigned subspecifically, primarily due to inadequate samples. On the Greater Antillean islands of Hispaniola, Cuba, and Puerto Rico is a complex of anoles which have come to be called" grass anoles" due to their habitat. The head and body shapes, as well as their very elongate tails, make these anoles conform to the shapes of blades of grass, although they are not rigidly restricted to this sort of physical situation. -

Distrito Nacional
DISTRITO NACIONAL enlace MYMAPS https://drive.google.com/open?id=16aUcqk1Boi8sMwNQJl9fWrjkUpylwggd&usp=sharing 37 ALBERGUES VÁLIDOS 26,623 personas Número de teléfono Capacidad del albergue Municipio donde se encuentra Código del albergue Nombre completo del albergue Nombre de la persona de contacto de la persona de según superficie cubierta Coordenada X Coordenada Y el albergue contacto (3,5m2/pers) Distrito Nacional DO-010002 Polideportivo San Carlos Luis Peña (809) 308-3411 274 personas -69.89178 18.47893 Santo Domingo de Guzmán DO-010007 Politécnico Don Bosco Modesto Cabrera (829) 699-3290 -69.901247 18.478692 Santo Domingo de Guzmán DO-010008 Parroquia Don Bosco Pedro Arias (809) 858-9928 -69.899758 18.479236 Santo Domingo de Guzmán DO-010010 Iglesia Abventista Del 7Mo Dia Franco Creor Pastor Reini (809) 904-7084 13,392 personas -69.889178 18.476092 Santo Domingo de Guzmán DO-010011 Parroquia San Carlos Borromeo Solangel De Los Santos (809) 689-5020 1,225 personas -69.893956 18.475336 Santo Domingo de Guzmán DO-010012 Asociación De Detallista Del Dn Maria Isabel Pichardo (829) 399-3360 178 personas -69.889074 18.479367 Santo Domingo de Guzmán DO-010014 Escuela Santo Socorro Sandra Morel (809) 856-9374 -69.893783 18.478761 Santo Domingo de Guzmán DO-010017 Escuela Básica/Primaria Republica De Hondura Anny Medrano (829) 654-1254 -69.888648 18.494045 Santo Domingo de Guzmán DO-010020 Escuela Santo Socorro Odalis Soriano (809) 919-5847 -69.888012 18.485820 Santo Domingo de Guzmán DO-010025 Liceo Panamericano Udelka Gonzales (829) 393-9835 -

FUNCIONARIOS OBLIGADOS POR LA LEY 311-14 QUE NO HAN PRESENTADO SU DECLARACIÓN JURADA DE PATRIMONIO Actualizado Al 2 De Agosto De 2016
OFICINA DE EVALUACIÓN Y FISCALIZACIÓN DEL PATRIMONIO DE LOS FUNCIONARIOS PÚBLICOS FUNCIONARIOS OBLIGADOS POR LA LEY 311-14 QUE NO HAN PRESENTADO SU DECLARACIÓN JURADA DE PATRIMONIO Actualizado al 2 de Agosto de 2016 2DO 2DO SECTOR (SI TIPO #. NOMBRE APELLIDO CARGO INSTITUCIÓN/ PROVINCIA NOMBRE APELLIDO APLICA) INSTITUCIÓN ADMINISTRADORA GENERAL DE BIENES 1 EMERSON SORIANO EX. ADMINSITRADOR GENERAL NACIONALES CENTRALIZADA 2 BELINDA CUEVAS SYLVAIN ENCARGADO DE COMPRAS ARCHIVO GENERAL DE LA NACION CENTRALIZADA ASTILLEROS NAVALES DE LA ARMADA DE 3 TIRSO DE LEON GERVACIO ENCARGADO DE COMPRAS LA REPÚBLICA DOMINICANA CENTRALIZADA AUTORIDAD METROPOLITANA DE 4 DAVID JOSE GOMEZ CASTRO COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 5 AQUILINO LEBRON CUELLO COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 6 MANUEL A GUZMAN HIDALGO COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 7 MILTON ODALIS DE LEON PEÑA COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 8 DANIEL RAMOS ALVAREZ COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 9 FRANCISCO DE JESUS PEREZ COLON COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 10 CAONABO CACERES MEJIA COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 11 LENIN ANTONIO JAQUEZ GONZALEZ COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 12 FREDERICK A. PERALTA NARANJO COMANDANTE TRANSPORTE (AMET) CENTRALIZADA AUTORIDAD METROPOLITANA DE 13 GREGORIO CANELA ROSARIO COMANDANTE -

Lista General Puestos Vacunacioì†N COVID-19 Del 29-03 Al 04-4-2021
Centros de vacunación contra COVID-19 funcionando del 29-03 al 04-04-2021 DPS Puesto de vacunación Dirección Fecha funcionamiento 1 CPN 3-4 Calle José Francisco Peña Gómez # 53, Pedernales 2 CPN 5-6 PedernalesCalle Proyecto # S/N, Sector Los Robles 29-03 al 04-04-2021 3 CPN 7-8 Centro del pueblo, Oviedo 4 UNAP 9 Juancho, Pederrnales Región de Salud VI DPS-DAS AZUA Vacunador puesto de vacunación No. Nombre puesto de vacunación Dirección Municipio Fecha de funcionamiento Nombre Teléfono 5 1 UNIVERSIDAD UTESUR C- HERNANDO GORJON. 34 Azua 26/03/2021 PETRONILA MERAN 809-335-0235 6 2 CENTRO COMUNAL BOSQUE SECO BARRIO LA COLONIA C- PERSEVERANCIA LA COLONIA ESPAÑOLA AZUA Azua 31/03/2021 LCDA. CARMEN FAMILIA 829-584-6377 7 3 UNAP LAS LOMAS CARRETERA PRINCIPAL Azua 05/04/2021 SONIA RAMIREZ 809-756-8157 8 4 CPN LOS JOVILLOS CARRETERA AZUA LA PLENA Azua 01/04/2021 SOBEIDA GARCIA 809-424-1899 9 5 UNAP CUCHILLA C- PADRE LORENZO SECTOR LA CUCHILLA Azua LCDA. SOCORO MERAN 849-3420149 10 6 UNAP TABARA ARRIBA C- DAMIAN PIMENTEL SECTOR LA CLINICA Azua 05/04/2021 DRA. ACELA SANCHEZ 829-448-6650 11 7 UNAP LAS LOMAS CALLE PRINCIPAL LAS LOMAS AZUA 02/04/2021 SONIA RAMIREZ 809-756-8157 12 8 ANSONIA CARRETERA PRINCIPAL AZUA LA PLENA AZUA 29/03/2021 DAMARIS TOREZ 809-924-1496 13 9 UNAP EL HIGUERO CARRETERA DUARTE PERALTA AZUA 02/04/2021 YDAISA RAMIREZ 809-249-5137 14 10 UNAP ESTEBANIA CARRETERA DUARTE, LA ENTRADA AZUA 02/05/2021 EVELYN SANCHEZ 809-967-2804 15 11 UNAP VILLA CORAZON CARRETERA AZUA PERALTA, CALLE PRINCIPAL AZUA 02/04/2021 DELIA MARGARITA PEREZ 809-235-2166 16 12 UNAP. -

Welcome Message from the President of Hotel and Tourism Association of the Dominican Republic, Inc
www.drdate.net www.drdate.net Welcome Message from the President of Hotel and Tourism Association of the Dominican Republic, Inc. ASONAHORES Dear colleagues and travel partners, I am delighted to welcome you to DATE 2016. This is our seventeenth show and as a result of year-to-year growth, our largest to date. Buyers have more choices since practically the entire tourism sector of the Dominican Republic is here represented. Thousands of appointments have been set in advance and more will take place during the event, making for two days full of great business opportunities. I would like to thank all of our partners who make this event possible especially the Ministry of Tourism of the Dominican Republic, Paradisus Palma Real Punta Cana, and Hard Rock Hotel Punta Cana, our host sponsors. Everyone at DATE 2016 hopes you enjoy successful days and looks forward to seeing you here again next year. Sincerely, Simón B. Suárez President ASOCIACIÓN DE HOTELES Y TURISMO DE LA REPÚBLICA DOMINICANA, INC. PRELIMINARY Schedule of Events The Schedule of Events is designed to provide the most flexible environment for conducting business in an efficient manner. Monday April 18th, 2016 6:00 pm Grand Opening of the V Collection by Viva Resorts at Samaná (Private Event) Tuesday April 19th, 2016 Official Arrival day for all DATE delegates. 10:00 am – 5:30 pm Credential Claiming and Supplier booth set-up 7:00 pm Welcome Reception - Hosted by Paradisus Palma Real Wednesday April 20th, 2016 7:30 am – 9:15 am Credential Claiming 9:15 am – 9:55 am Buyer to Supplier -

Works, and Natural History
INSECTA MUNDI A Journal of World Insect Systematics 0182 Antlions of Hispaniola (Neuroptera: Myrmeleontidae) Robert B. Miller Research Associate Florida State Collection of Arthropods P. O. Box 147100 Gainesville, FL, 32614-7100, U.S.A. Lionel A. Stange Florida Department of Agriculture and Consumer Services Florida State Collection of Arthropods P. O. Box 147100 Gainesville, FL, 32614-7100, U.S.A. Date of Issue: May 27, 2011 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Robert B. Miller and Lionel A. Stange Antlions of Hispaniola (Neuroptera: Myrmeleontidae) Insecta Mundi 0182: 1-28 Published in 2011 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 U. S. A. http://www.centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. Topics considered for publication include systematics, taxonomy, nomencla- ture, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book re- views or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources including the Zoological Record, CAB Abstracts, etc. Insecta Mundi is published irregularly throughout the year, with completed manu- scripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. One author of each submitted manu- script must be a current member of the Center for Systematic Entomology. -

(Asfls)CORRESPONDIENTE AL PRESUPUESTO DEL AÑO 2013
TRANSFERENCIAS CORRIENTES A LAS ASOCIACIONES SIN FINES DE LUCRO (ASFLs)CORRESPONDIENTE AL PRESUPUESTO Página 1 de 46 DEL AÑO 2013(VALORES EN RD$) ENTIDAD CODIGO DE PRESUPUESTO CAP NOMBRE RECEPTORA BENEFICIARIO 2013 MINISTERIO DE LA PRESIDENCIA 0201 9998 401509962 ACCION COMUNITARIA POR EL PROGRESO, SANTO DOMINGO 300,000.00 0201 9998 401512955 ACCION NACIONAL PRO-COMUNITARIA, INC., D. N. 320,000.00 0201 9998 430098914 ACCION SOLIDARIA PARA LA PROVINCIA DE BAHORUCO 300,000.00 0201 9992 411013446 ACTIVIDAD PRO-ENSEÑANZA DOMINICANA, INC., SAN PEDRO DE MACORIS 300,000.00 0201 9998 43004431 AGENCIA DE DESARROLLO ECONOMICO LOCAL DE MONTE PLATA 900,000.00 0201 9998 409000948 AGENCIA DE DESARROLLO ECONOMICO LOCAL DE VALVERDE, (ADELVA) 300,000.00 0201 9998 407000578 AGENCIA DE DESARROLLO LOCAL, SALCEDO 1,300,000.00 0201 9998 417007191 AGENCIA PROVINCIAL DE DESARROLLO DE PEDERNALES , INC. 700,000.00 0201 9998 430011436 ALBERGUE DIVINA PROVIDENCIA DE LA VICTORIA, SANTO DOMINGO N. 900,000.00 0201 9991 430007951 ALBERGUE DIVINA PROVIDENCIA SANTIAGO 600,000.00 0201 9992 430019194 ALBERGUE EDUCATIVO INFANTIL, MOCA, ESPAILLAT 240,000.00 0201 9998 430080039 ALBERGUE INFANTIL SANTA ROSA DE LIMA, SANTO DOMINGO N. 240,000.00 0201 9998 423002468 ALBERGUE NACIONAL PARA IMPEDIDOS FISICOS, SANTO DOMINGO N. 480,000.00 0201 9998 430022799 ALBERGUE VILLA ESPERANZA DE AZUA 153,600.00 0201 9991 430016209 ALIANZA SOLIDARIA PARA LA LUCHA CONTRA EL VIH/SIDA, INC. 120,000.00 0201 9998 401511932 ASOCIACION ACCION COMUNITARIA HERMANAS MIRABAL. INC., D. N. 600,000.00 0201 -

Pediobius Cajanus Sp. N. (Hymenoptera
JHR 45: 41–54 (2015) Pediobius cajanus sp. n. (Hymenoptera, Eulophidae)... 41 doi: 10.3897/JHR.45.4964 RESEARCH ARTICLE http://jhr.pensoft.net Pediobius cajanus sp. n. (Hymenoptera, Eulophidae), an important natural enemy of the Asian fly (Melanagromyza obtusa (Malloch)) (Diptera, Agromyzidae) in the Dominican Republic Rosina Taveras1, Christer Hansson2,3 1 Laboratorio Control Biologico Facultad de Ciencias Agronomicas y Veterinarias de la Universidad Autonoma de Santo Domingo 2 Scientific Associate, the Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom 3 Museum of Biology (Entomology), Lund University, Lund, Sweden Corresponding author: Christer Hansson ([email protected]) Academic editor: Hannes Baur | Received 24 March 2015 | Accepted 28 May 2015 | Published 7 September 2015 http://zoobank.org/367A2222-F7E5-477D-9893-A3D0158902EC Citation: Taveras R, Hansson C (2015) Pediobius cajanus sp. n. (Hymenoptera, Eulophidae), an important natural enemy of the Asian fly (Melanagromyza obtusa (Malloch)) (Diptera, Agromyzidae) in the Dominican Republic. Journal of Hymenoptera Research 45: 41–54. doi: 10.3897/JHR.45.4964 Abstract Pediobius cajanus sp. n. is described based on material from the Dominican Republic, where it is wide- spread, and it is anticipated to have a much larger distribution in tropical America. It is compared to other species of Pediobius from the New World, and is also compared to P. vignae (Risbec), a similar species from Africa with similar biology. The new species is a gregarious endoparasitoid of the pupae of the Asian fly, Melanagromyza obtusa (Malloch), an agromyzid that causes major damage to pigeon pea, Cajanus cajan (L). Millspauh. In the Dominican Republic P. -

Hurricane Irma
DREF Final Report Dominican Republic: Hurricane Irma DREF Operation MDRDO010 Glide n° TC-2017-000125-DOM Date of issue: 25 June 2018 Date of the disaster: 6 September 2017 Head of operation (responsible for this operation): Point of contact (name and title): Gustavo Lara – Pabel Angeles - Regional Management Disaster Coordinator for South America - IFRC Director General – Dominican Red Cross (DRC) Expected timeframe: 4 months; End date 6 January Start date for the operation: 6 September 2017 2018 Overall operations budget: 450,614 Swiss francs (CHF) No. of people to be assisted: 2,096 families Number of people affected: 1,300,000 (10,480 people) National Society Presence (No. of volunteers, personnel, branches): The Dominican Red Cross has 1 national headquarters, 187 branches and 20,000 volunteers Partners of the Red Cross Red Crescent actively involved in the operation (if available and relevant): International Federation of Red Cross and Red Crescent Societies (IFRC) Country Cluster Office in Haiti – Pan American Disaster Response Unit (PADRU). Support is also being provided to the National Society through the Spanish Red Cross and the Canadian Red Cross Society’s Capacity Building for Emergency Response Project (CERA), which is co-funded by the Canadian government. Other partner organizations actively involved in the operation: National Civil Defence, Ministry of Health, Ministry of Agriculture, Ministry of Education, Ministry of Housing (INVI for its acronym in Spanish), National Emergency Operation Centre (NEOC), Ministry of Public Works, municipalities, Ministry of Defence, United Nations Office for the Coordination of Humanitarian Affairs (UN-OCHA). National Emergency Commission (NEC for its acronym in Spanish), Presidency of the Republic, National Institute of Potable Water and Sewerage (INAPA for its acronym in Spanish), Oxfam, World Vision. -

JULIO Fedomu:Layout 1.Qxd
FEDOMUFEDOMUEn Marcha AÑO 1, NO.2, JULIO 2009, SANTO DOMINGO, REPUBLICA DOMINICANA EL DESARROLLO DE UN PAÍS SE INICIA EN LOS MUNICIPIOS Programa Nacional de Asfaltado en el limbo Pags. 2 y 3 Lamentable y preocupante decisión de la Suprema. Pag. 3 FEDOMU orienta sobre seguridad social. Pag. 4 Comunicadores dominicanos se capacitan Pag. 10 en San Salvador en Asuntos Municipales. Síndicos muestran buenas prácticas. Pag. 11 Avanzan en eleboracion del Reglamento Ley 176-07. Pag. 5 Visite nuestra página web: www.fedomu.org.do Página 2 /Julio 2009 Sólo en anuncio se convirtió “Plan Nacional de Asfaltado” Los Secretarios de Estado de Interior y Po- ción los suministraran a los medios de comu- licía y Obras Públicas, doctor Franklin Al- nicación, cosa que a la fecha no ha sucedido. meyda Rancier y el ingeniero Víctor Díaz En sus promesas, aseguró que después Rúa, recibieron en el despacho del primero a de iniciar los trabajos de asfaltado en la región una comisión de la Federación Dominicana de Norte, continuarán con las regiones Sur y Municipios (FEDOMU), encabezada por su Este, informó el funcionario, dijo que el plan in- Presidente, doctor José Reyes, cuyo principal volucrará a todos los síndicos del país sin im- punto de agenda fue el inicio del prometido portar el partido al que pertenezcan, lo que se amplio programa de asfaltado. ha quedado en promesa. El doctor Franklin Almeyda Rancier informó En aquel esperanzador encuentro (en en esa ocasión que su participación en el en- principio), participaron además de los Se- cuentro es de servir como “facilitador” o enlace cretarios de Interior y Policía Doctor Fran- entre el Gobierno Central frente a FEDOMU y klin Almeyda Rancier; el ingeniero Víctor el Secretario de Obras Públicas, Ing.