Acquired, Drug-Induced Long QT Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The QT Interval: How Long Is Too Long?
heart matters The QT interval: How long is too long? By Natalie K. Cox, RN Staff Nurse • Coronary Care Unit • McLeod Regional Medical Center • Florence, S.C. What’s the QT interval and why’s it so the appearance that the QT interval is pro- important? In this article, I’ll answer these longed. However, a wide QRS complex questions plus show you how to measure represents depolarization, and LQTS is a dis- the QT interval and how to recognize the order of repolarization. Sometimes the end types of long QT syndrome (LQTS) and of a T wave isn’t clearly defi ned, which can their symptoms, causes, and treatments. make it diffi cult to get the QT measurement. Irregular rhythms also make it diffi cult to The long and short of it obtain a consistent QT interval measure- The QT interval on the ECG is measured ment. For example, atrial fi brillation makes from the beginning of the QRS complex to it extremely diffi cult to measure a QT inter- the end of the T wave (see ECG components). val because fi nding a T wave isn’t always It represents the time it takes for the ventricles of the heart to depolarize and repolarize, or to ECG components contract and relax. The QT interval is longer when First positive deflection after the the heart rate is slower and short- ECG components P or Q wave er when the heart rate is faster. So it’s necessary to calculate the corrected QT interval (QTc) using the Bazett formula: QT interval divided by the square root of the R-R interval. -

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

Stroke Mimics and Chameleons: Quandaries in the Field
Stroke Mimics and Chameleons: Quandaries in the Field Madeleine Geraghty, MD Rockwood Multicare What’s the difference Stroke mimic: Looks like a stroke, is something else Stroke chameleon: Looks like something else, is really a stroke! Scope of the Mimic Recent eval by Briard, et al: ◦ 960 patients transported by EMS during an 18 month period ◦ 42% mimics 55% other neurologic diagnoses 20% seizures, 19% migraines, 11% peripheral neuropathies 45% non-neurologic diagnoses Cardiac 16%, psychiatric 12%, infectious 9% ◦ Neurologic mimics were younger (~64 years) than non-neurologic mimics (~70 years) Entering a new era Large vessel occlusions Now a 24 hour time window for mechanical thrombectomy ◦ Most centers will likely activate the > 6 hour patients from within the ED, still working out those details Volume of stroke mimics/chameleons in the new time window? Effects on resource management? ◦ At the hospital level? ◦ At the regional level with distance transports? Need Emergency Responder Impressions now more than ever in order to learn for the future!! General Principles Positive symptoms Indicate an excess of central nervous system neuron electrical discharges Visual: flashing lights, zig zag shapes, lines, shapes, objects sensory: paresthesia, pain motor: jerking limb movements Migraine, Seizure are characterized with having “positive” symptoms Negative symptoms Indicate a loss or reduction of central nervous system neuron function – loss of vision, hearing, sensation, limb power. TIA/Stroke present with “negative” symptoms. -

Long QT Syndrome: from Channels to Cardiac Arrhythmias
Long QT syndrome: from channels to cardiac arrhythmias Arthur J. Moss, Robert S. Kass J Clin Invest. 2005;115(8):2018-2024. https://doi.org/10.1172/JCI25537. Review Series Long QT syndrome, a rare genetic disorder associated with life-threatening arrhythmias, has provided a wealth of information about fundamental mechanisms underlying human cardiac electrophysiology that has come about because of truly collaborative interactions between clinical and basic scientists. Our understanding of the mechanisms that control the critical plateau and repolarization phases of the human ventricular action potential has been raised to new levels through these studies, which have clarified the manner in which both potassium and sodium channels regulate this critical period of electrical activity. Find the latest version: https://jci.me/25537/pdf Review series Long QT syndrome: from channels to cardiac arrhythmias Arthur J. Moss1 and Robert S. Kass2 1Heart Research Follow-up Program, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA. 2Department of Pharmacology, Columbia University Medical Center, New York, New York, USA. Long QT syndrome, a rare genetic disorder associated with life-threatening arrhythmias, has provided a wealth of information about fundamental mechanisms underlying human cardiac electrophysiology that has come about because of truly collaborative interactions between clinical and basic scientists. Our understanding of the mecha- nisms that control the critical plateau and repolarization phases of the human ventricular action potential has been raised to new levels through these studies, which have clarified the manner in which both potassium and sodium channels regulate this critical period of electrical activity. -

Anemia and the QT Interval in Hypertensive Patients
2084 International Journal of Collaborative Research on Internal Medicine & Public Health Anemia and the QT interval in hypertensive patients Ioana Mozos 1*, Corina Serban 2, Rodica Mihaescu 3 1 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 2 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 3 1st Department of Internal Medicine, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania * Corresponding Author ; Email: [email protected] Abstract Introduction: A prolonged ECG QT interval duration and an increased QT dispersion (QTd) are predictors of sudden cardiac death. Anemia is known as a marker of adverse outcome in cardiovascular disease. Objective: The aim was to assess the relationship between anemia and QT intervals in hypertensive patients. Method: A total of 72 hypertensive patients underwent standard 12-lead ECG. QT intervals and QT dispersions were manually measured. Complete blood count was also assessed. Result: Linear regression analysis revealed significant associations between prolonged QTc and increased QTd and anemia and macrocytosis, respectively. Multiple regression analysis revealed a significant association between red cell distribution width (RDW) >15% and prolonged heart rate corrected maximal QT interval duration (QTc) and QT interval in lead DII (QTIIc). The most sensitive and specific predictor of prolonged QTc and QTIIc was anisocytosis. Anemia was the most sensitive predictor of -
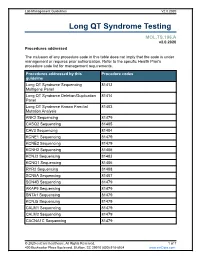
Long QT Syndrome Testing
Lab Management Guidelines V2.0.2020 Long QT Syndrome Testing MOL.TS.196.A v2.0.2020 Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline Long QT Syndrome Sequencing 81413 Multigene Panel Long QT Syndrome Deletion/Duplication 81414 Panel Long QT Syndrome Known Familial 81403 Mutation Analysis ANK2 Sequencing 81479 CASQ2 Sequencing 81405 CAV3 Sequencing 81404 KCNE1 Sequencing 81479 KCNE2 Sequencing 81479 KCNH2 Sequencing 81406 KCNJ2 Sequencing 81403 KCNQ1 Sequencing 81406 RYR2 Sequencing 81408 SCN5A Sequencing 81407 SCN4B Sequencing 81479 AKAP9 Sequencing 81479 SNTA1 Sequencing 81479 KCNJ5 Sequencing 81479 CALM1 Sequencing 81479 CALM2 Sequencing 81479 CACNA1C Sequencing 81479 © 2020 eviCore healthcare. All Rights Reserved. 1 of 7 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V2.0.2020 What is Long QT syndrome Definition Long QT Syndrome (LQTS) is caused by mutations in a number of genes, most of which are related to the functioning of sodium or potassium ion channels in the heart.1 Testing may offer prognostic information in some cases, as specific genes and even specific mutations within those genes may have some correlation to risk for sudden death, effectiveness of beta-blocker therapy, and preventive strategies.1-4 Signs and symptoms of long QT syndrome (LQTS) are variable, but may include a prolonged QT interval on an electrocardiogram, torsades de pointes, syncope, seizures, cardiac arrest, and sudden cardiac death.1,2 Many patients with LQTS can be largely asymptomatic, with cardiac arrest or sudden cardiac death as the first and only symptom. -

Pacemaker Syndrome Pacemaker Therapy Has Become an Important Therapeutic Option for Patients with Heart Rhythm Conditions Worldwide
360 Cardiology Pacemaker syndrome Pacemaker therapy has become an important therapeutic option for patients with heart rhythm conditions worldwide. Te number of elderly patients needing pacemakers is on the increase due to an ageing population worldwide. Pacemaker syndrome consists of the cardiovascular signs and symptoms of heart failure and hypotension induced by right ventricular (RV) pacing. Dr Satnam Singh Research Registrar, University of Aberdeen, Level 3, Polwarth building, Aberdeen email [email protected] Pacemaker syndrome is a term syndrome occurring in dual trial was a single blinded study proposed in 1979 by Erbel and chamber modes.5,6 It can even enrolling around 2000 patients refers to symptoms and signs in occur with AAI pacing with long with sick sinus syndrome. the pacemaker patient caused by PR intervals. All patients were implanted inadequate timing of atrial and dual chamber pacemakers ventricular contractions.1 It was first programmed to VVIR or DDDR described in 1969 by Mitsui et al2 as Incidence before implantation. Pacemaker an iatrogenic disease characterised syndrome was a secondary by the disappearance of symptoms Te overall incidence of pacemaker endpoint studied. Severe with restoration of atrioventricular syndrome is very difficult to pacemaker syndrome developed synchrony (AV synchrony). estimate but is about 20% in a in nearly 20% of VVIR-paced It means if atria and ventricles landmark trial called the Mode patients and improved with contract at appropriate timings (as Selection Trial (MOST).7 It occurs reprogramming to the dual- close to physiological), pacemaker with equal frequency in both sexes chamber pacing mode. syndrome can be prevented. -

Syncope Fainting, Or Syncope, Is the Sudden Loss of Consciousness and Ability to Stand
Northwestern Memorial Hospital Patient Education CONDITIONS AND DISEASES Syncope Fainting, or syncope, is the sudden loss of consciousness and ability to stand. It is also called “passing out.” This common If you have any problem is the cause of many falls and injuries. One third of people faint at least once during their life. Syncope may occur questions, ask without warning or can be signaled by: ■ Feelings of weakness your physician ■ Feeling hot or sweating ■ Dizziness or nurse. ■ Visual changes ■ Nausea ■ Palpitations Causes of syncope Fainting is due to a sudden decrease in blood flow and oxygen to the brain. There are many causes of syncope, but most fall into 1 of 3 major types. Abnormal nerve reflex Nerves that control heart rate, blood pressure and other body functions may respond in an abnormal way and cause syncope. This can be triggered by: ■ Standing ■ Pain ■ Unpleasant sight or smell ■ Stress, anxiety or emotional distress ■ Coughing, sneezing or swallowing ■ Urinating or having a bowel movement Fainting due to an abnormal nerve reflex is more likely to occur under certain conditions, such as dehydration, viral infection, after prolonged bed rest or a lack of sleep or regular food intake. Types of syncope that involve an abnormal nerve reflex include: ■ Vasovagal (neurocardiac) syncope is the most common type and can occur at any age. It often occurs when blood pools in the leg veins. This triggers a reflex where the heart rate, blood pressure or both may suddenly fall. It generally is not a dangerous condition and can be prevented by avoiding situations that can trigger syncope. -

Neurotransmitter Actions
Central University of South Bihar Panchanpur, Gaya, India E-Learning Resources Department of Biotechnology NB: These materials are taken/borrowed/modified/compiled from various resources like research articles and freely available internet websites, and are meant to be used solely for the teaching purpose in a public university, and for serving the needs of specified educational programmes. Dr. Jawaid Ahsan Assistant Professor Department of Biotechnology Central University of South Bihar (CUSB) Course Code: MSBTN2003E04 Course Name: Neuroscience Neurotransmitter Actions • Excitatory Action: – A neurotransmitter that puts a neuron closer to an action potential (facilitation) or causes an action potential • Inhibitory Action: – A neurotransmitter that moves a neuron further away from an action potential • Response of neuron: – Responds according to the sum of all the neurotransmitters received at one time Neurotransmitters • Acetylcholine • Monoamines – modified amino acids • Amino acids • Neuropeptides- short chains of amino acids • Depression: – Caused by the imbalances of neurotransmitters • Many drugs imitate neurotransmitters – Ex: Prozac, zoloft, alcohol, drugs, tobacco Release of Neurotransmitters • When an action potential reaches the end of an axon, Ca+ channels in the neuron open • Causes Ca+ to rush in – Cause the synaptic vesicles to fuse with the cell membrane – Release the neurotransmitters into the synaptic cleft • After binding, neurotransmitters will either: – Be destroyed in the synaptic cleft OR – Taken back in to surrounding neurons (reuptake) Excitable cells: Definition: Refers to the ability of some cells to be electrically excited resulting in the generation of action potentials. Neurons, muscle cells (skeletal, cardiac, and smooth), and some endocrine cells (e.g., insulin- releasing pancreatic β cells) are excitable cells. -

Drugs and Life-Threatening Ventricular Arrhythmia Risk: Results from the DARE Study Cohort
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2017-016627 on 16 October 2017. Downloaded from Drugs and life-threatening ventricular arrhythmia risk: results from the DARE study cohort Abigail L Coughtrie,1,2 Elijah R Behr,3,4 Deborah Layton,1,2 Vanessa Marshall,1 A John Camm,3,4,5 Saad A W Shakir1,2 To cite: Coughtrie AL, Behr ER, ABSTRACT Strengths and limitations of this study Layton D, et al. Drugs and Objectives To establish a unique sample of proarrhythmia life-threatening ventricular cases, determine the characteristics of cases and estimate ► The Drug-induced Arrhythmia Risk Evaluation study arrhythmia risk: results from the the contribution of individual drugs to the incidence of DARE study cohort. BMJ Open has allowed the development of a cohort of cases of proarrhythmia within these cases. 2017;7:e016627. doi:10.1136/ proarrhythmia. Setting Suspected proarrhythmia cases were referred bmjopen-2017-016627 ► These cases have provided crucial safety by cardiologists across England between 2003 and 2011. information, as well as underlying clinical and ► Prepublication history for Information on demography, symptoms, prior medical and genetic data. this paper is available online. drug histories and data from hospital notes were collected. ► Only patients who did not die as a result of the To view these files please visit Participants Two expert cardiologists reviewed data the journal online (http:// dx. doi. proarrhythmia could be included. for 293 referred cases: 130 were included. Inclusion org/ 10. 1136/ bmjopen- 2017- ► Referral of cases by cardiologists alone may have criteria were new onset or exacerbation of pre-existing 016627). -

Atrial Fibrillation (ATRIA) Study
European Journal of Human Genetics (2014) 22, 297–306 & 2014 Macmillan Publishers Limited All rights reserved 1018-4813/14 www.nature.com/ejhg REVIEW Atrial fibrillation: the role of common and rare genetic variants Morten S Olesen*,1,2,4, Morten W Nielsen1,2,4, Stig Haunsø1,2,3 and Jesper H Svendsen1,2,3 Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1–2% of the general population. A number of studies have demonstrated that AF, and in particular lone AF, has a substantial genetic component. Monogenic mutations in lone and familial AF, although rare, have been recognized for many years. Presently, mutations in 25 genes have been associated with AF. However, the complexity of monogenic AF is illustrated by the recent finding that both gain- and loss-of-function mutations in the same gene can cause AF. Genome-wide association studies (GWAS) have indicated that common single-nucleotide polymorphisms (SNPs) have a role in the development of AF. Following the first GWAS discovering the association between PITX2 and AF, several new GWAS reports have identified SNPs associated with susceptibility of AF. To date, nine SNPs have been associated with AF. The exact biological pathways involving these SNPs and the development of AF are now starting to be elucidated. Since the first GWAS, the number of papers concerning the genetic basis of AF has increased drastically and the majority of these papers are for the first time included in a review. In this review, we discuss the genetic basis of AF and the role of both common and rare genetic variants in the susceptibility of developing AF. -

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1.