Importance of Forests Outside Protected Area Networks for Large
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Great Hornbill Buceros Bicornis, Wreathed Hornbill Aceros Undulatus and Oriental Pied Hornbill Anthracoceros Albirostris
Bird Conservation International (2004) 14:S39–S52. BirdLife International 2004 doi:10.1017/S0959270905000213 Printed in the United Kingdom Nest-site selection and nesting success of three hornbill species in Arunachal Pradesh, north-east India: Great Hornbill Buceros bicornis, Wreathed Hornbill Aceros undulatus and Oriental Pied Hornbill Anthracoceros albirostris APARAJITA DATTA and G. S. RAWAT Summary Nest-site selection by the sympatric Great Hornbill Buceros bicornis, Wreathed Hornbill Aceros undulatus and Oriental Pied Hornbill Anthracoceros albirostris was investigated in a lowland tropical forest in Arunachal Pradesh, north-east India during 1997–2000. Infor- mation on two nests of Rufous-necked Hornbill Aceros nipalensis in higher-elevation forests is also presented. All species nested in live trees of three tree genera, 83% (n = 36) in Tetrameles nudiflora, an emergent deciduous softwood, relatively common in lowland foothill forests. No difference was recorded in nest-tree species or nesting habitats of sympatric hornbills, but there were a few differences in structural characteristics of nest- trees. Cavity size was the main variable separating the three species. Great Hornbills used larger cavities while Oriental Pied Hornbills used smaller cavities closer to riverine areas. Nesting was attempted at 64% of known sites and successful fledging of chicks was 80% overall (n = 72 nests, pooled over 4 years). Nest-trees in disturbed habitats near human habitation were used but were often abandoned or unsuccessful and 50% of all nest-trees were inactive by the end of the study. Potential large nest-trees had a density of 5.9/ha, that of the two most used species was 1.3/ha, and minimum nest densities of all three species was about 1 pair/km2. -

EIA & EC for Kathalchari Field Development, Block
EIA & EC for Kathalchari Field Development, Block (AA-ONN-2002/1), Tripura Final EIA Report Prepared for: Jubilant Oil and Gas Private Limited Prepared by: SENES Consultants India Pvt. Ltd. June, 2016 EIA for development activities of hydrocarbon, installation of GGS & pipeline laying at Kathalchari FINAL REPORT EIA & EC for Kathalchari Field Development, Block (AA-ONN-2002/1), Tripura M/s Jubilant Oil and Gas Private Limited For on and behalf of SENES Consultants India Ltd Approved by Mr. Mangesh Dakhore Position held NABET-QCI Accredited EIA Coordinator for Offshore & Onshore Oil and Gas Development and Production Date 28.12.2015 Approved by Mr. Sunil Gupta Position held NABET-QCI Accredited EIA Coordinator for Offshore & Onshore Oil and Gas Development and Production Date February 2016 The EIA report preparation have been undertaken in compliance with the ToR issued by MoEF vide letter no. J-11011/248/2013-IA II (I) dated 28th January, 2014. Information and content provided in the report is factually correct for the purpose and objective for such study undertaken. SENES/M-ESM-20241/June, 2016 i JOGPL EIA for development activities of hydrocarbon, installation of GGS & pipeline laying at Kathalchari INFORMATION ABOUT EIA CONSULTANTS Brief Company Profile This Environmental Impact Assessment (EIA) report has been prepared by SENES Consultants India Pvt. Ltd. SENES India, registered with the Companies Act of 1956 (Ranked No. 1 in 1956), has been operating in the county for more than 11 years and holds expertise in conducting Environmental Impact Assessments, Social Impact Assessments, Environment Health and Safety Compliance Audits, Designing and Planning of Solid Waste Management Facilities and Carbon Advisory Services. -

Athirapally Hydel Electric Project
Athirapally Hydel Electric Project drishtiias.com/printpdf/athirapally-hydel-electric-project Why in News Recently, the Kerala government has approved the proposed Athirapally Hydro Electric Project (AHEP) on the Chalakudy river in Thrissur district of the state. There are already five dams for power and one for irrigation and it will be the seventh along the 145 km course of the Chalakudy river. Chalakudy River It originates in the Anamalai region of Tamil Nadu and is joined by its major tributaries Parambikulam, Kuriyarkutti, Sholayar, Karapara and Anakayam in Kerala. The river flows through Palakkad, Thrissur and Ernakulam districts of Kerala. It is the 4th longest river in Kerala and one of very few rivers of Kerala, which is having relics of riparian vegetation in substantial level. A riparian zone is the interface between land and a river or stream. Plant habitats and communities along the river margins and banks are called riparian vegetation, characterized by hydrophilic plants. It is the richest river in fish diversity perhaps in India as it contains 85 species of freshwater fishes out of the 152 species known from Kerala only. The famous waterfalls, Athirappilly Falls and Vazhachal Falls, are situated on this river. It merges with the Periyar River near Puthenvelikkara in Ernakulam district. Key Points 1/3 The total installed capacity of AHEP is 163 MW and the project is supposed to make use of the tail end water coming out of the existing Poringalkuthu Hydro Electric Project that is constructed across the Chalakudy river. AHEP envisages diverting water from the Poringalkuthu project as well as from its own catchment of 26 sq km. -

Bandhavgarh Tiger Reserve (BM)
India – Bandhavgarh Tiger Reserve (BM) Naturetrek Tour Report 12 - 21 March 2010 Report compiled by Himanshu Rathore Naturetrek Cheriton Mill Cheriton Alresford Hampshire SO24 0NG England T: +44 (0)1962 733051 F: +44 (0)1962 736426 E: [email protected] W: www.naturetrek.co.uk Tour Report India - Bandhavgarh Tiger Reserve (BM) Tour leader: Himanshu Rathore Participants: Martin Baggot Kate Baggot Helen Prandy Tracey Hart Barbara Wild Summary It was an amazingly successful tour - tigers were literally appearing from behind every bush, and we saw two male tigers fight which is a sight that makes the heart shiver with growling noises they make. Other than the tigers we had great views from the fort and seeing the vultures hover at eye level was exciting. It was overall a great safari experience. Day 0 Friday 12th March Travel from the UK Day 1 Saturday 13th March Having met at the airport, we checked into the ever-so-stylish Ashok Country Hotel where there was time to wash and change and have a hearty meal. We then set out for the station to catch the train. Day 2 Sunday 14th March Our train rolled into the Katni junction at 4.50am. We were met by our driver at the station and set out on a beautiful journey to Bandhavgarh National Park, travelling through beautiful forests and great sceneries. On arrival at the camp we got a much-deserved cup of tea and some hot breakfast to go with it. After breakfast we took a short walk around the camp just to get our orientation. -

Metabolitos Secundarios Obtenidos De La Familia Myristicaceae Que Producen Inhibición Enzimática Y Actividad Biológica
METABOLITOS SECUNDARIOS OBTENIDOS DE LA FAMILIA MYRISTICACEAE QUE PRODUCEN INHIBICIÓN ENZIMÁTICA Y ACTIVIDAD BIOLÓGICA Xiomara Alejandra Cabrera Martínez Universidad Nacional de Colombia Facultad de Ciencias, Departamento de Química Bogotá D.C. Colombia 2019 METABOLITOS SECUNDARIOS OBTENIDOS DE LA FAMILIA MYRISTICACEAE QUE PRODUCEN INHIBICIÓN ENZIMÁTICA Y ACTIVIDAD BIOLÓGICA Xiomara Alejandra Cabrera Martínez Tesis o trabajo de investigación presentada(o) como requisito parcial para optar al título de: Magister en Ciencias - Química Director: Qco. M.Sc. Dr. Sc. Luis Enrique Cuca Suarez Línea de Investigación: Química de Productos Naturales Grupo de Investigación: Estudio Químico y de Actividad Biológica de Rutaceae y Myristicaceae Colombianas Universidad Nacional de Colombia Facultad de Ciencias, Departamento de Química Bogotá D.C. Colombia 2019 Mi estrella que brilla en el cielo y guía mis caminos. Agradecimientos A Dios por la vida y permitirme vivir este proceso de formación. A toda mi familia por su apoyo, especialmente a mi padre. A mi director y profesor Luis Enrique Cuca Suárez quien me acepto en este proyecto, y en el grupo de Investigación. Gracias por su orientación, dedicación y sabiduría, por el tiempo y compromiso de asesoramiento en el desarrollo de este trabajo. A los profesores del grupo de investigación de productos naturales de la Universidad Nacional de Colombia por cada uno de sus aportes. A mis compañeras de maestría por sus colaboraciones. A la Universidad Nacional de Colombia por recibirme en sus aulas y permitirme lograr esta meta. Resumen y Abstract IX Resumen Diferentes especies de la familia Myristicaceae han sido utilizadas con fines medicinales, nutricionales e industriales, mostrando así la importancia y potencial de la familia en diversos campos. -

An Ecosystem Paradigm for Vasant Vihar, New Delhi, India Janaki Turaga
TURAGA: Urban birds–New Delhi 85 Birds and trees in an urban context: An ecosystem paradigm for Vasant Vihar, New Delhi, India Janaki Turaga Turaga, J., 2015. Birds and trees in an urban context: An ecosystem paradigm for Vasant Vihar, New Delhi, India. Indian BIRDS 10 (3&4): 85–93. Janaki Turaga, G 5, Phase 1, New Palam Vihar, Gurgaon 122017, Haryana, India. E-mail: [email protected] Manuscript received on 19 August 2013. Introduction observations throughout the year over varying times and Avian diversity, and density, in and around urban conglomerations durations. have been the focus of birdwatchers in a selective manner. Bird The primary areas of the study were the avenue trees, followed watching in urban areas has largely been confined, but not by major parks in the colony, sidewalk gardens of the houses, limited, to areas that are either protected, or still remain largely and trees in home gardens (observed from the roadside). Palam Marg, which marks the outer peripheral boundary of Vasant Vihar, natural, such as protected areas, wetlands, ornamental parks, was covered from beginning of the intersection of Palam Marg etc., where it is relatively easy to observe large numbers of and Nelson Mandela Road, right upto Paschimi Marg of Vasant diverse birds. Sporadic reports of birds observed in residential or Vihar. Along this road not only avenue plantations, and avenue commercial urban areas have been reported. However, there is trees, but also the central verge (the divider between the two absence of data on avifauna supported by a micro-urban habitat, lanes of the road), which was planted with vegetation, and where such as, a residential colony, commercial area, office complex, birds nested, were covered. -

A CONCISE REPORT on BIODIVERSITY LOSS DUE to 2018 FLOOD in KERALA (Impact Assessment Conducted by Kerala State Biodiversity Board)
1 A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) Editors Dr. S.C. Joshi IFS (Rtd.), Dr. V. Balakrishnan, Dr. N. Preetha Editorial Board Dr. K. Satheeshkumar Sri. K.V. Govindan Dr. K.T. Chandramohanan Dr. T.S. Swapna Sri. A.K. Dharni IFS © Kerala State Biodiversity Board 2020 All rights reserved. No part of this book may be reproduced, stored in a retrieval system, tramsmitted in any form or by any means graphics, electronic, mechanical or otherwise, without the prior writted permission of the publisher. Published By Member Secretary Kerala State Biodiversity Board ISBN: 978-81-934231-3-4 Design and Layout Dr. Baijulal B A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) EdItorS Dr. S.C. Joshi IFS (Rtd.) Dr. V. Balakrishnan Dr. N. Preetha Kerala State Biodiversity Board No.30 (3)/Press/CMO/2020. 06th January, 2020. MESSAGE The Kerala State Biodiversity Board in association with the Biodiversity Management Committees - which exist in all Panchayats, Municipalities and Corporations in the State - had conducted a rapid Impact Assessment of floods and landslides on the State’s biodiversity, following the natural disaster of 2018. This assessment has laid the foundation for a recovery and ecosystem based rejuvenation process at the local level. Subsequently, as a follow up, Universities and R&D institutions have conducted 28 studies on areas requiring attention, with an emphasis on riverine rejuvenation. I am happy to note that a compilation of the key outcomes are being published. -

School of Health Sciences Newsletter August 2014 CONTENTS HEAD OF
School of Health Sciences Newsletter August 2014 CONTENTS Head of School Report Presentations (Teaching and Research) Did you know? Research News UniSA – PAFC Official Launch Publications by Staff and Students Staff Appointments and News School Administration Teaching and Learning HEAD OF SCHOOL Hi All Welcome to the August Newsletter. Staff Appointments I am pleased to report the appointments of Sandy Maranna as Lecturer in Medical Sonography; Cathy Cookson as Lecturer in Medical Sonography; Caroline Fryer and Emily Ward as Lecturers in Physiotherapy; Michael Dale as Lecturer in Human Movement and Narelle Korotkov as Academic Services Officer (Health Sciences and Occupational Therapy). Port Adelaide Football Club (PAFC) Partnership Launch TV personality and President of the club (David Koch) and Professor David Lloyd (Vice-Chancellor) presided over the PAFC partnership launch, with distinguished guests including Hon Tom Kenyon MP and Hon Susan Close MP, and Dr Ian Gould (Chancellor), Sir Eric Neal and representatives of SA’s leading sports organisations, commercial partners, stakeholders and friends of PAFC and UniSA. The University has a long relationship with PAFC which includes a sports science PhD scholarship, sponsored annual student prizes, cadetships, UniSA support for the annual Aboriginal Cup carnival and the Gavin Wanganeen Indigenous scholarship. The launch marked the signing of a MoU in June to form a high performance partnership centred on research and education in elite sport, a commitment to community engagement to provide effective communication to remote Aboriginal communities and the development of strategies to explore compatible connections in China and Asia. Among the exciting announcements made by David Koch and David Lloyd, were plans to launch a High Performance MSc preceded by a pilot program of two modules in 2015, development of a UniSA scholarship to conduct research in relation to PAFC’s WillPower program into communities in the APY lands and a scholarship to a student from a university in China to work with PAFC and UniSA. -

Medicinal Plants Research
V O L U M E -III Glimpses of CCRAS Contributions (50 Glorious Years) MEDICINAL PLANTS RESEARCH CENTRAL COUNCIL FOR RESEARCH IN AYURVEDIC SCIENCES Ministry of AYUSH, Government of India New Delhi Illllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllll Glimpses of CCRAS contributions (50 Glorious years) VOLUME-III MEDICINAL PLANTS RESEARCH CENTRAL COUNCIL FOR RESEARCH IN AYURVEDIC SCIENCES Ministry of AYUSH, Government of India New Delhi MiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiM Illllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllll © Central Council for Research in Ayurvedic Sciences Ministry of AYUSH, Government of India, New Delhi - 110058 First Edition - 2018 Publisher: Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, Government of India, New Delhi, J. L. N. B. C. A. H. Anusandhan Bhavan, 61-65, Institutional Area, Opp. D-Block, Janakpuri, New Delhi - 110 058, E-mail: [email protected], Website : www.ccras.nic.in ISBN : 978-93-83864-27-0 Disclaimer: All possible efforts have been made to ensure the correctness of the contents. However Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, shall not be accountable for any inadvertent error in the content. Corrective measures shall be taken up once such errors are brought -

KERALA STATE ELECTRICITY BOARD on Athirappilly HE Project -163 MW Western Ghats Ecology Expert Panel (WGEEP)
KERALA STATE ELECTRICITY BOARD Presentation on Athirappilly HE Project -163 MW before Western Ghats Ecology Expert Panel (WGEEP) Chalakudy, Thrissur – 29 th January 2011 Athirappilly HE Project – 163 MW Athirappilly HEP ––LocationLocation Proposed project is located in Chalakudy river basin in Thrissur District, Kerala State Cascade Scheme with 94 % utilization of tail race discharges and spills from existing upstream reservoirs Projects up stream Sholayar Hydro Electric Project (54MW) Poringalkuthu Hydro Electric Project (48MW) KERALA STATE ELECTRICITY BOARD Athirappilly HE Project – 163 MW An overview Athirappilly project KERALA STATE ELECTRICITY BOARD Athirappilly HE Project – 163 MW Surge Dam Reservoir Tunnel Dam toe Power House Power House Vertical shaft Kannankuzhy thodu Kannankuzhythodu LONGITUDINAL SECTION Poringal dam Charpa Poringal Power Houses Poringal Adit Reservoir Tunnel Athirappilly Reservoir Dam toe PH To Sholayar Surge SH 21 Pokalappara Colony Dam Power House(2X80) MW Replacement Road To Chalakudy Athirappilly Falls Chalakudy River LAY OUT PLAN Athirappilly HE Project – 163 MW • The upstream most project is Sholayar which is operation since 1966. • The Sholayar project utilizes the water resources of Sholayar River a tributary of Chalakudy River • The tail water of Sholayar after generation flows to downstream Poringalkuthu reservoir. • The Poringalkuthu project is in operation for more than 50 years it is in the main river itself. Athirappilly HE Project – 163 MW • The tail water of Poringalkuthu discharges to Chalakudy -
List of Lacs with Local Body Segments (PDF
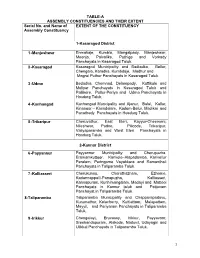
TABLE-A ASSEMBLY CONSTITUENCIES AND THEIR EXTENT Serial No. and Name of EXTENT OF THE CONSTITUENCY Assembly Constituency 1-Kasaragod District 1 -Manjeshwar Enmakaje, Kumbla, Mangalpady, Manjeshwar, Meenja, Paivalike, Puthige and Vorkady Panchayats in Kasaragod Taluk. 2 -Kasaragod Kasaragod Municipality and Badiadka, Bellur, Chengala, Karadka, Kumbdaje, Madhur and Mogral Puthur Panchayats in Kasaragod Taluk. 3 -Udma Bedadka, Chemnad, Delampady, Kuttikole and Muliyar Panchayats in Kasaragod Taluk and Pallikere, Pullur-Periya and Udma Panchayats in Hosdurg Taluk. 4 -Kanhangad Kanhangad Muncipality and Ajanur, Balal, Kallar, Kinanoor – Karindalam, Kodom-Belur, Madikai and Panathady Panchayats in Hosdurg Taluk. 5 -Trikaripur Cheruvathur, East Eleri, Kayyur-Cheemeni, Nileshwar, Padne, Pilicode, Trikaripur, Valiyaparamba and West Eleri Panchayats in Hosdurg Taluk. 2-Kannur District 6 -Payyannur Payyannur Municipality and Cherupuzha, Eramamkuttoor, Kankole–Alapadamba, Karivellur Peralam, Peringome Vayakkara and Ramanthali Panchayats in Taliparamba Taluk. 7 -Kalliasseri Cherukunnu, Cheruthazham, Ezhome, Kadannappalli-Panapuzha, Kalliasseri, Kannapuram, Kunhimangalam, Madayi and Mattool Panchayats in Kannur taluk and Pattuvam Panchayat in Taliparamba Taluk. 8-Taliparamba Taliparamba Municipality and Chapparapadavu, Kurumathur, Kolacherry, Kuttiattoor, Malapattam, Mayyil, and Pariyaram Panchayats in Taliparamba Taluk. 9 -Irikkur Chengalayi, Eruvassy, Irikkur, Payyavoor, Sreekandapuram, Alakode, Naduvil, Udayagiri and Ulikkal Panchayats in Taliparamba -

Myristicaceae
BLUMEA 42 (1997) 111-190 Notes on Southeast Asian and Malesian Myristica and description of new taxa (Myristicaceae). With keys arranged per geographical area (New Guinea excepted) W.J.J.O. de Wilde Rijksherbarium/Hortus Botanicus, P.O. Box 9514, 2300 RA Leiden, The Netherlands Summary Following the introductory sections, a general key, and regional keys, noteworthy observations are whole distributional of the of given for selected species of Myristica covering the area genus west New Guinea. New taxa, i.e. species (14), subspecies (10), and varieties (2) are fully described and annotated. All accepted names are arranged alphabetically, followed by an index. Contents Introduction 112 References 112 Acknowledgements 113 General key for Continental Southeast Asia, Malesia, and Australia (excl. New Guinea and the Pacific) 113 Regional key for Continental Southeast Asia 123 Regional key for West Malesia 124 Regional key for East Malesia (most of New Guinea excepted) 128 Enumeration of the partial areas and concerning keys 133 1. India, Sri Lanka (with partial keys) 133 2. Continental Southeast Asia 134 3. Malay Peninsula and Singapore 134 4. Sumatra 135 5. Java (with general key to areas 3, 4 & 5) 135 6. Borneo (with regional key) 138 7. Philippines (with regional key) 141 8. Sulawesi (with regional key) 144 9. Moluccas (incl. Aru Islands) 145 10. Lesser Sunda Islands 145 List of annotated or described and accepted taxa, newly alphabetically arranged . 146 Index 189 112 BLUMEA —Vol. 42, No. 1, 1997 Introduction With the completion of the revision of all Myristica material in the Leiden collection, with extension to most of the materials of the Kew herbarium and incidental loans of important collections of other herbaria, quite a number of new taxa were still to be published.