An Update on the Genetics of Ocular Coloboma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Study of Microphthalmia, Anophthalmia, and Coloboma (MAC
16 ORIGINAL ARTICLE J Med Genet: first published as 10.1136/jmg.39.1.16 on 1 January 2002. Downloaded from National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology D Morrison, D FitzPatrick, I Hanson, K Williamson, V van Heyningen, B Fleck, I Jones, J Chalmers, H Campbell ............................................................................................................................. J Med Genet 2002;39:16–22 We report an epidemiological and genetic study attempting complete ascertainment of subjects with microphthalmia, anophthalmia, and coloboma (MAC) born in Scotland during a 16 year period beginning on 1 January 1981. A total of 198 cases were confirmed giving a minimum live birth preva- lence of 19 per 100 000. One hundred and twenty-two MAC cases (61.6%) from 115 different fami- See end of article for lies were clinically examined and detailed pregnancy, medical, and family histories obtained. A authors’ affiliations simple, rational, and apparently robust classification of the eye phenotype was developed based on ....................... the presence or absence of a defect in closure of the optic (choroidal) fissure. A total of 85/122 Correspondence to: (69.7%) of cases had optic fissure closure defects (OFCD), 12/122 (9.8%) had non-OFCD, and Dr D FitzPatrick, MRC 25/122 (20.5%) had defects that were unclassifiable owing to the severity of the corneal or anterior Human Genetics Unit, chamber abnormality. Segregation analysis assuming single and multiple incomplete ascertainment, Western General Hospital, respectively, returned a sib recurrence risk of 6% and 10% in the whole group and 8.1% and 13.3% Edinburgh EH4 2XU, UK; in the OFCD subgroup. -

Investigating Cone Photoreceptor Development Using Patient-Derived NRL Null Retinal Organoids
ARTICLE https://doi.org/10.1038/s42003-020-0808-5 OPEN Investigating cone photoreceptor development using patient-derived NRL null retinal organoids Alyssa Kallman1,11, Elizabeth E. Capowski 2,11, Jie Wang 3, Aniruddha M. Kaushik4, Alex D. Jansen2, Kimberly L. Edwards2, Liben Chen4, Cynthia A. Berlinicke3, M. Joseph Phillips2,5, Eric A. Pierce6, Jiang Qian3, ✉ ✉ Tza-Huei Wang4,7, David M. Gamm2,5,8 & Donald J. Zack 1,3,9,10 1234567890():,; Photoreceptor loss is a leading cause of blindness, but mechanisms underlying photoreceptor degeneration are not well understood. Treatment strategies would benefit from improved understanding of gene-expression patterns directing photoreceptor development, as many genes are implicated in both development and degeneration. Neural retina leucine zipper (NRL) is critical for rod photoreceptor genesis and degeneration, with NRL mutations known to cause enhanced S-cone syndrome and retinitis pigmentosa. While murine Nrl loss has been characterized, studies of human NRL can identify important insights for human retinal development and disease. We utilized iPSC organoid models of retinal development to molecularly define developmental alterations in a human model of NRL loss. Consistent with the function of NRL in rod fate specification, human retinal organoids lacking NRL develop S- opsin dominant photoreceptor populations. We report generation of two distinct S-opsin expressing populations in NRL null retinal organoids and identify MEF2C as a candidate regulator of cone development. 1 Institute of Genetic Medicine, Johns Hopkins University School of Medicine, Baltimore, USA. 2 Waisman Center, University of Wisconsin-Madison, Madison, USA. 3 Department of Ophthalmology, Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, USA. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Supplemental Materials ZNF281 Enhances Cardiac Reprogramming
Supplemental Materials ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression Huanyu Zhou, Maria Gabriela Morales, Hisayuki Hashimoto, Matthew E. Dickson, Kunhua Song, Wenduo Ye, Min S. Kim, Hanspeter Niederstrasser, Zhaoning Wang, Beibei Chen, Bruce A. Posner, Rhonda Bassel-Duby and Eric N. Olson Supplemental Table 1; related to Figure 1. Supplemental Table 2; related to Figure 1. Supplemental Table 3; related to the “quantitative mRNA measurement” in Materials and Methods section. Supplemental Table 4; related to the “ChIP-seq, gene ontology and pathway analysis” and “RNA-seq” and gene ontology analysis” in Materials and Methods section. Supplemental Figure S1; related to Figure 1. Supplemental Figure S2; related to Figure 2. Supplemental Figure S3; related to Figure 3. Supplemental Figure S4; related to Figure 4. Supplemental Figure S5; related to Figure 6. Supplemental Table S1. Genes included in human retroviral ORF cDNA library. Gene Gene Gene Gene Gene Gene Gene Gene Symbol Symbol Symbol Symbol Symbol Symbol Symbol Symbol AATF BMP8A CEBPE CTNNB1 ESR2 GDF3 HOXA5 IL17D ADIPOQ BRPF1 CEBPG CUX1 ESRRA GDF6 HOXA6 IL17F ADNP BRPF3 CERS1 CX3CL1 ETS1 GIN1 HOXA7 IL18 AEBP1 BUD31 CERS2 CXCL10 ETS2 GLIS3 HOXB1 IL19 AFF4 C17ORF77 CERS4 CXCL11 ETV3 GMEB1 HOXB13 IL1A AHR C1QTNF4 CFL2 CXCL12 ETV7 GPBP1 HOXB5 IL1B AIMP1 C21ORF66 CHIA CXCL13 FAM3B GPER HOXB6 IL1F3 ALS2CR8 CBFA2T2 CIR1 CXCL14 FAM3D GPI HOXB7 IL1F5 ALX1 CBFA2T3 CITED1 CXCL16 FASLG GREM1 HOXB9 IL1F6 ARGFX CBFB CITED2 CXCL3 FBLN1 GREM2 HOXC4 IL1F7 -
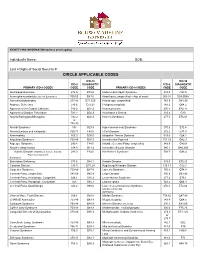
Circle Applicable Codes
IDENTIFYING INFORMATION (please print legibly) Individual’s Name: DOB: Last 4 Digits of Social Security #: CIRCLE APPLICABLE CODES ICD-10 ICD-10 ICD-9 DIAGNOSTIC ICD-9 DIAGNOSTIC PRIMARY ICD-9 CODES CODE CODE PRIMARY ICD-9 CODES CODE CODE Abetalipoproteinemia 272.5 E78.6 Hallervorden-Spatz Syndrome 333.0 G23.0 Acrocephalosyndactyly (Apert’s Syndrome) 755.55 Q87.0 Head Injury, unspecified – Age of onset: 959.01 S09.90XA Adrenaleukodystrophy 277.86 E71.529 Hemiplegia, unspecified 342.9 G81.90 Arginase Deficiency 270.6 E72.21 Holoprosencephaly 742.2 Q04.2 Agenesis of the Corpus Callosum 742.2 Q04.3 Homocystinuria 270.4 E72.11 Agenesis of Septum Pellucidum 742.2 Q04.3 Huntington’s Chorea 333.4 G10 Argyria/Pachygyria/Microgyria 742.2 Q04.3 Hurler’s Syndrome 277.5 E76.01 or 758.33 Aicardi Syndrome 333 G23.8 Hyperammonemia Syndrome 270.6 E72.4 Alcohol Embryo and Fetopathy 760.71 F84.5 I-Cell Disease 272.2 E77.0 Anencephaly 655.0 Q00.0 Idiopathic Torsion Dystonia 333.6 G24.1 Angelman Syndrome 759.89 Q93.5 Incontinentia Pigmenti 757.33 Q82.3 Asperger Syndrome 299.8 F84.5 Infantile Cerebral Palsy, unspecified 343.9 G80.9 Ataxia-Telangiectasia 334.8 G11.3 Intractable Seizure Disorder 345.1 G40.309 Autistic Disorder (Childhood Autism, Infantile 299.0 F84.0 Klinefelter’s Syndrome 758.7 Q98.4 Psychosis, Kanner’s Syndrome) Biotinidase Deficiency 277.6 D84.1 Krabbe Disease 333.0 E75.23 Canavan Disease 330.0 E75.29 Kugelberg-Welander Disease 335.11 G12.1 Carpenter Syndrome 759.89 Q87.0 Larsen’s Syndrome 755.8 Q74.8 Cerebral Palsy, unspecified 343.69 G80.9 -

2018 Etiologies by Frequencies
2018 Etiologies in Order of Frequency by Category Hereditary Syndromes and Disorders Count CHARGE Syndrome 958 Down syndrome (Trisomy 21 syndrome) 308 Usher I syndrome 252 Stickler syndrome 130 Dandy Walker syndrome 119 Cornelia de Lange 102 Goldenhar syndrome 98 Usher II syndrome 83 Wolf-Hirschhorn syndrome (Trisomy 4p) 68 Trisomy 13 (Trisomy 13-15, Patau syndrome) 60 Pierre-Robin syndrome 57 Moebius syndrome 55 Trisomy 18 (Edwards syndrome) 52 Norrie disease 38 Leber congenital amaurosis 35 Chromosome 18, Ring 18 31 Aicardi syndrome 29 Alstrom syndrome 27 Pfieffer syndrome 27 Treacher Collins syndrome 27 Waardenburg syndrome 27 Marshall syndrome 25 Refsum syndrome 21 Cri du chat syndrome (Chromosome 5p- synd) 16 Bardet-Biedl syndrome (Laurence Moon-Biedl) 15 Hurler syndrome (MPS I-H) 15 Crouzon syndrome (Craniofacial Dysotosis) 13 NF1 - Neurofibromatosis (von Recklinghausen dis) 13 Kniest Dysplasia 12 Turner syndrome 11 Usher III syndrome 10 Cockayne syndrome 9 Apert syndrome/Acrocephalosyndactyly, Type 1 8 Leigh Disease 8 Alport syndrome 6 Monosomy 10p 6 NF2 - Bilateral Acoustic Neurofibromatosis 6 Batten disease 5 Kearns-Sayre syndrome 5 Klippel-Feil sequence 5 Hereditary Syndromes and Disorders Count Prader-Willi 5 Sturge-Weber syndrome 5 Marfan syndrome 3 Hand-Schuller-Christian (Histiocytosis X) 2 Hunter Syndrome (MPS II) 2 Maroteaux-Lamy syndrome (MPS VI) 2 Morquio syndrome (MPS IV-B) 2 Optico-Cochleo-Dentate Degeneration 2 Smith-Lemli-Opitz (SLO) syndrome 2 Wildervanck syndrome 2 Herpes-Zoster (or Hunt) 1 Vogt-Koyanagi-Harada -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

Deletion of Vax1 from Gonadotropin-Releasing Hormone (Gnrh) Neurons Abolishes Gnrh Expression and Leads to Hypogonadism and Infertility
3506 • The Journal of Neuroscience, March 23, 2016 • 36(12):3506–3518 Cellular/Molecular Deletion of Vax1 from Gonadotropin-Releasing Hormone (GnRH) Neurons Abolishes GnRH Expression and Leads to Hypogonadism and Infertility Hanne M. Hoffmann,1 Crystal Trang,1 Ping Gong,1 Ikuo Kimura,2 Erica C. Pandolfi,1 and XPamela L. Mellon1 1Department of Reproductive Medicine and the Center for Reproductive Science and Medicine, University of California, San Diego, La Jolla, California 92093-0674, and 2Department of Applied Biological Science, Graduate School of Agriculture, Tokyo University of Agriculture and Technology, Fuchu-shi 183-8509, Japan Hypothalamic gonadotropin-releasing hormone (GnRH) neurons are at the apex of the hypothalamic-pituitary-gonadal axis that regu- lates mammalian fertility. Herein we demonstrate a critical role for the homeodomain transcription factor ventral anterior homeobox 1 (VAX1) in GnRH neuron maturation and show that Vax1 deletion from GnRH neurons leads to complete infertility in males and females. Specifically, global Vax1 knock-out embryos had normal numbers of GnRH neurons at 13 d of gestation, but no GnRH staining was detected by embryonic day 17. To identify the role of VAX1 specifically in GnRH neuron development, Vax1flox mice were generated and lineage tracing performed in Vax1flox/flox:GnRHcre:RosaLacZ mice. This identified VAX1 as essential for maintaining expression of Gnrh1. The absence of GnRH staining in adult Vax1flox/flox:GnRHcre mice led to delayed puberty, hypogonadism, and infertility. To address the mechanism by which VAX1 maintains Gnrh1 transcription, the capacity of VAX1 to regulate Gnrh1 transcription was evaluated in the GnRH cell lines GN11 and GT1-7. -

Genetic and Developmental Basis of Renal Coloboma (Papillorenal) Syndrome
Perspective Genetic and developmental basis of renal coloboma (papillorenal) syndrome Expert Rev. Ophthalmol. 4(2), 135–xxx (2009) Lisa A Schimmenti Renal coloboma syndrome, also known as papillorenal syndrome, is characterized by optic Department of Pediatrics and nerve anomalies and kidney hypodysplasia. Autosomal dominant mutations in the gene Ophthalmology, University of encoding the paired box transcription factor PAX2 can be identified in nearly half of all Minnesota Medical School, 420 patients with this phenotype. The primary ophthalmologic findings include congenital central Delaware Street Southeast, retinal vasculature absence associated with abnormalities in retinal blood vessel patterning MMC 730, Minnesota, and deeply excavated optic discs. Other published findings include optic nerve hypoplasia, MN 55455, USA optic nerve cyst, optic nerve pits, retinal coloboma, microphthalmia and scleral staphyloma. Tel.: +1 612 624 5613 Visual acuity ranges from normal to severe impairment. Up to one third of affected patients Fax: +1 612 626 2993 will develop end-stage renal disease. Mouse and zebrafish withPax2/pax2a mutations provide [email protected] developmentally based explanations for the observed phenotypic observations in affected patients. KEYWORDS: optic nerve coloboma • optic nerve dysplasia • papillorenal syndrome • PAX2 • renal coloboma syndrome The clinical presentation of optic nerve anomalies total of 10 years later, Weaver et al. reported a case associated with renal hypodysplasia should alert the of two brothers, both with optic nerve colobomas, clinician to the possibility that a patient may have interstitial nephritis and renal failure [3]. Weaver renal coloboma syndrome, a condition also known noted the similarity of the optic nerve colobomas as papillorenal syndrome (OMIM#120330). The in his patients with the ‘morning glory anomaly’, optic nerve findings could be described as a ‘dys- previously reported by Karcher et al. -

Level Estimates of Maternal Smoking and Nicotine Replacement Therapy During Pregnancy
Using primary care data to assess population- level estimates of maternal smoking and nicotine replacement therapy during pregnancy Nafeesa Nooruddin Dhalwani BSc MSc Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy November 2014 ABSTRACT Background: Smoking in pregnancy is the most significant preventable cause of poor health outcomes for women and their babies and, therefore, is a major public health concern. In the UK there is a wide range of interventions and support for pregnant women who want to quit. One of these is nicotine replacement therapy (NRT) which has been widely available for retail purchase and prescribing to pregnant women since 2005. However, measures of NRT prescribing in pregnant women are scarce. These measures are vital to assess its usefulness in smoking cessation during pregnancy at a population level. Furthermore, evidence of NRT safety in pregnancy for the mother and child’s health so far is nebulous, with existing studies being small or using retrospectively reported exposures. Aims and Objectives: The main aim of this work was to assess population- level estimates of maternal smoking and NRT prescribing in pregnancy and the safety of NRT for both the mother and the child in the UK. Currently, the only population-level data on UK maternal smoking are from repeated cross-sectional surveys or routinely collected maternity data during pregnancy or at delivery. These obtain information at one point in time, and there are no population-level data on NRT use available. As a novel approach, therefore, this thesis used the routinely collected primary care data that are currently available for approximately 6% of the UK population and provide longitudinal/prospectively recorded information throughout pregnancy. -

CHARGE Syndrome
orphananesthesia Anaesthesia recommendations for CHARGE syndrome Disease name: CHARGE syndrome ICD 10: Q87.8 Synonyms: CHARGE association; Hall-Hittner syndrome Disease summary: CHARGE syndrome was initially defined as a non-random association of anomalies: - Coloboma - Heart defect - Atresia choanae (choanal atresia) - Retarded growth and development - Genital hypoplasia - Ear anomalies/deafness In 1998, an expert group defined the major (the classical 4C´s: Choanal atresia, Coloboma, Characteristic ear and Cranial nerve anomalies) and minor criteria of CHARGE syndrome [1]. In 2004, mutations in the CHD7 gene were identified as the major cause. The inheritance pattern is autosomal dominant with variable expressivity. Almost all mutations occurs de novo, but parent-to-child transmission has occasionally been reported [2]. Clinical criteria for CHARGE syndrome [1] Major criteria: • Coloboma • Choanal Atresia • Cranial nerve anomalies • Abnormalities of the inner, middle, or external ear Minor criteria: • Cardiaovascular malformations • Genital hypoplasia or delayed pubertal development • Cleft lip and/or palate • Tracheoesophageal defects • Distinctive CHARGE facies • Growth retardation • Developmental delay Occasional: • Renal anomalies: duplex system, vesicoureteric reflux • Spinal anomalies: scoliosis, osteoporosis • Hand anomalies 1 • Neck/shoulder anomalies • Immune system disorders Individuals with all four major characteristics or three major and three minor characteristics are highly likely to have CHARGE syndrome [1]. CHARGE syndrome -

CHARGE Factsheet 3 Clinical Diagnosis and Features
The Information Pack CHARGE for Practitioners Factsheet 3 CHARGE syndrome: major and minor medical diagnostic criteria plus later onset features DR JEREMY KIRK, MD, FRCP, FRCPCH, Consultant Paediatric Endocrinologist, Birmingham Children’s Hospital Original diagnostic criteria The initial association of coloboma and choanal atresia with other congenital abnormalities was first described by Hall and separately Hittner et al. in 1979 (Hall, 1979; Hittner et al., 1979). In 1981 there was further description and expansion of the condition (Pagon et al., 1981). It was at this stage that the acronym CHARGE (C–coloboma, H–heart disease, A–atresia choanae, R–retarded growth and retarded development and/or CNS anomalies, G–genital hypoplasia, and E–ear anomalies and/or deafness) was made. In order to make the diagnosis of CHARGE syndrome, historically four out of six of the features of the acronym needed to be fulfilled, although one should be either choanal atresia or a coloboma (Pagon et al., 1981). From association to syndrome been several attempts to refine the diagnostic criteria, Initially CHARGE was described as an association; namely by Blake et al. (1998) and Verloes (2005). Both a nonrandom collection of birth defects, rather than of these use major features which are very specific for a syndrome, which is a more recognisable pattern of CHARGE syndrome, along with other minor features. birth defects (often with a known genetic cause). With the identification of the gene CHD7 in 2004 (Vissers The criteria suggested by Blake et al. consist of four et al., 2004) it has now been renamed a syndrome, major ‘C’s: as CHD7 is mutated in at least 60% of patients with 1.