6.04 the Biological Pump C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Four Hundred Million Years of Silica Biomineralization in Land Plants
Four hundred million years of silica biomineralization in land plants Elizabeth Trembath-Reicherta,1, Jonathan Paul Wilsonb, Shawn E. McGlynna,c, and Woodward W. Fischera aDivision of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125; bDepartment of Biology, Haverford College, Haverford, PA 19041; and cGraduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji-shi, Tokyo 192-0397, Japan Edited by Thure E. Cerling, University of Utah, Salt Lake City, UT, and approved February 20, 2015 (received for review January 7, 2015) Biomineralization plays a fundamental role in the global silicon Silica is widely used within plants for structural support and cycle. Grasses are known to mobilize significant quantities of Si in pathogen defense (19–21), but it remains a poorly understood the form of silica biominerals and dominate the terrestrial realm aspect of plant biology. Recent work on the angiosperm Oryza today, but they have relatively recent origins and only rose to sativa demonstrated that silica accumulation is facilitated by taxonomic and ecological prominence within the Cenozoic Era. transmembrane proteins expressed in root cells (21–24). Phy- This raises questions regarding when and how the biological silica logenetic analysis revealed that these silicon transport proteins cycle evolved. To address these questions, we examined silica were derived from a diverse family of modified aquaporins that abundances of extant members of early-diverging land plant include arsenite and glycerol transporters (19, 21, 25, 26). A clades, which show that silica biomineralization is widespread different member of this aquaporin family was recently identi- across terrestrial plant linages. Particularly high silica abundances fied that enables silica uptake in the horsetail Equisetum,an are observed in lycophytes and early-diverging ferns. -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Require
Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jorge Andres Villa Betancur Graduate Program in Environmental Science The Ohio State University 2014 Dissertation Committee: William J. Mitsch, Co-advisor Gil Bohrer, Co-advisor James Bauer Jay Martin Copyrighted by Jorge Andres Villa Betancur 2014 Abstract Emission and uptake of greenhouse gases and the production and transport of dissolved organic matter in different wetland plant communities are key wetland functions determining two important ecosystem services, climate regulation and nutrient cycling. The objective of this dissertation was to study the variation of methane emissions, carbon sequestration and exports of dissolved organic carbon in wetland plant communities of a subtropical climate in south Florida. The plant communities selected for the study of methane emissions and carbon sequestration were located in a natural wetland landscape and corresponded to a gradient of inundation duration. Going from the wettest to the driest conditions, the communities were designated as: deep slough, bald cypress, wet prairie, pond cypress and hydric pine flatwood. In the first methane emissions study, non-steady-state rigid chambers were deployed at each community sequentially at three different times of the day during a 24- month period. Methane fluxes from the different communities did not show a discernible daily pattern, in contrast to a marked increase in seasonal emissions during inundation. All communities acted at times as temporary sinks for methane, but overall were net -2 -1 sources. Median and mean + standard error fluxes in g CH4-C.m .d were higher in the deep slough (11 and 56.2 + 22.1), followed by the wet prairie (9.01 and 53.3 + 26.6), bald cypress (3.31 and 5.54 + 2.51) and pond cypress (1.49, 4.55 + 3.35) communities. -

SHELLS in ACID Adapted from NAMEPA’S an Educator’S Guide to the Marine Environment: Shells in Acid
SHELLS IN ACID Adapted from NAMEPA’s An Educator’s Guide to the Marine Environment: Shells in Acid PURPOSE Students will test the strength of normal seashells versus shells that have been soaked in vinegar to simulate the weakening effect of ocean acidification. Students identify the correlation between decreasing oceanic pH (ocean acidification) and the weakening of shells and discuss the effect this could have on the health of shellfish in the world’s oceans. MATERIALS (PER GROUP OF 4) • *white vinegar • *small, thin seashells • *non-reactive containers (glass beakers, Pyrex, measuring glass) • *water • heavy books (several) • paper towels For #6: • shells (1 per student) • snack size plastic bags (1 per student) • Small amount of vinegar • magnifying glass *Before beginning this activity, shells should be pre-soaked overnight in a 1:1 solution of vinegar and fresh water. PROCEDURE 1. Engage/Elicit Ask the students to give examples of different species of shellfish. Answers may include clams, oysters, mussels, scallops, etc. Ask students why and where they have seen these creatures. Students’ knowledge may come from eating seafood, or perhaps from having seen them in an aquarium, a marina or in coastal areas. Ask the students why these animals are important to the marine environment and to human beings. 2. Explore Lay out an assemblage of the non-soaked shells. Have the students observe the shells. Allow the students to handle the shells and ask them why the development of shells is advantageous to such animals. Explain that shellfish are invertebrates, meaning that instead of having an internal skeleton like humans, invertebrates produce a hard, protective covering. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

From Nano-Gels to Marine Snow: a Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean
gels Review From Nano-Gels to Marine Snow: A Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean Antonietta Quigg 1,* , Peter H. Santschi 2 , Adrian Burd 3, Wei-Chun Chin 4 , Manoj Kamalanathan 1, Chen Xu 2 and Kai Ziervogel 5 1 Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA; [email protected] 2 Department of Marine and Coastal Environmental Science, Texas A&M University at Galveston, Galveston, TX 77553, USA; [email protected] (P.H.S.); [email protected] (C.X.) 3 Department of Marine Science, University of Georgia, Athens, GA 30602, USA; [email protected] 4 Department of Bioengineering, University of California, Merced, CA 95343, USA; [email protected] 5 Institute for the Study of Earth, Oceans and Space, University of New Hampshire, Durham, NH 03824, USA; [email protected] * Correspondence: [email protected] Abstract: Marine gels (nano-, micro-, macro-) and marine snow play important roles in regulating global and basin-scale ocean biogeochemical cycling. Exopolymeric substances (EPS) including transparent exopolymer particles (TEP) that form from nano-gel precursors are abundant materials in the ocean, accounting for an estimated 700 Gt of carbon in seawater. This supports local microbial communities that play a critical role in the cycling of carbon and other macro- and micro-elements in Citation: Quigg, A.; Santschi, P.H.; the ocean. Recent studies have furthered our understanding of the formation and properties of these Burd, A.; Chin, W.-C.; Kamalanathan, materials, but the relationship between the microbial polymers released into the ocean and marine M.; Xu, C.; Ziervogel, K. -

Murphey Et Al. 2019 Best Practices in Mitigation Paleontology
PROCEEDINGS of the San Diego Society of Natural History Founded 1874 Number 47 1 May 2019 BEST PRACTICES IN MITIGATION PALEONTOLOGY By Paul C. Murphey Paleo Solutions, 2785 Speer Boulevard, Suite 1, Denver, CO 80211, U.S.A.; [email protected]; Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Department of Earth Sciences, Denver Museum of Nature and Science, 2001 Colorado Boulevard, Denver, CO 80201, U.S.A. Georgia E. Knauss SWCA Environmental Consultants, 1892 S. Sheridan Avenue, Sheridan, WY 82801 U.S.A.; [email protected] Lanny H. Fisk PaleoResource Consultants, 550 High Street, Suite 108, Auburn, CA 95603, U.S.A. (deceased) Thomas A. Deméré Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Robert E. Reynolds Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] For correspondence, write to: Paul C. Murphey, Paleo Solutions, 4614 Lonespur Ct. Oceanside, CA 92056 Email: [email protected] [email protected] bpmp-19-01-fm Page 2 PDF Created: 2019-4-12: 9:20:AM 2 Paul C. Murphey, Georgia E. Knauss, Lanny H. Fisk, Thomas A. Deméré, and Robert E. Reynolds TABLE OF CONTENTS Abstract . 4 Introduction . 4 History and Scientific Contributions . 5 History of Mitigation Paleontology in the United States . 5 Methods Best Practice Categories . 7 1. Qualifications. 7 Confusion between Resource Disciplines . 7 Professional Geologists as Mitigation Paleontologists. 8 Mitigation Paleontologist Categories . -

Trends in Soil Solution Dissolved Organic Carbon (DOC) Concentrations Across European Forests
Biogeosciences, 13, 5567–5585, 2016 www.biogeosciences.net/13/5567/2016/ doi:10.5194/bg-13-5567-2016 © Author(s) 2016. CC Attribution 3.0 License. Trends in soil solution dissolved organic carbon (DOC) concentrations across European forests Marta Camino-Serrano1, Elisabeth Graf Pannatier2, Sara Vicca1, Sebastiaan Luyssaert3,a, Mathieu Jonard4, Philippe Ciais3, Bertrand Guenet3, Bert Gielen1, Josep Peñuelas5,6, Jordi Sardans5,6, Peter Waldner2, Sophia Etzold2, Guia Cecchini7, Nicholas Clarke8, Zoran Galic´9, Laure Gandois10, Karin Hansen11, Jim Johnson12, Uwe Klinck13, Zora Lachmanová14, Antti-Jussi Lindroos15, Henning Meesenburg13, Tiina M. Nieminen15, Tanja G. M. Sanders16, Kasia Sawicka17, Walter Seidling16, Anne Thimonier2, Elena Vanguelova18, Arne Verstraeten19, Lars Vesterdal20, and Ivan A. Janssens1 1Research Group of Plant and Vegetation Ecology, Department of Biology, University of Antwerp, Universiteitsplein 1, B-2610 Wilrijk, Belgium 2WSL, Swiss Federal Institute for Forest, Snow and Landscape Research, Zürcherstrasse 111, 8903, Birmensdorf, Switzerland 3Laboratoire des Sciences du Climat et de l’Environnement, LSCE/IPSL, CEA-CNRS-UVSQ, Université Paris-Saclay, 91191 Gif-sur-Yvette, France 4UCL-ELI, Université catholique de Louvain, Earth and Life Institute, Croix du Sud 2, 1348 Louvain-la-Neuve, Belgium 5CREAF, Cerdanyola del Vallès, 08193, Catalonia, Spain 6CSIC, Global Ecology Unit CREAF-CSIC-UAB, Cerdanyola del Vallès, 08193, Catalonia, Spain 7Department of Earth Sciences, University of Florence, Via La Pira 4, 50121 Florence, -
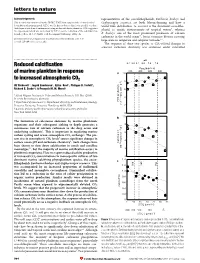
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Deciphering Ocean Carbon in a Changing World PERSPECTIVE Mary Ann Morana,1, Elizabeth B
PERSPECTIVE Deciphering ocean carbon in a changing world PERSPECTIVE Mary Ann Morana,1, Elizabeth B. Kujawinskib,1, Aron Stubbinsc,1, Rob Fatlandd,1, Lihini I. Aluwiharee, Alison Buchanf, Byron C. Crumpg, Pieter C. Dorresteinh,i,j, Sonya T. Dyhrmank,l, Nancy J. Hessm, Bill Howen, Krista Longneckerb, Patricia M. Medeirosa, Jutta Niggemanno, Ingrid Obernostererp, Daniel J. Repetab, and Jacob R. Waldbauerq Edited by David M. Karl, University of Hawaii, Honolulu, HI, and approved February 11, 2016 (received for review October 21, 2015) Dissolved organic matter (DOM) in the oceans is one of the largest pools of reduced carbon on Earth, comparable in size to the atmospheric CO2 reservoir. A vast number of compounds are present in DOM, and they play important roles in all major element cycles, contribute to the storage of atmospheric CO2 in the ocean, support marine ecosystems, and facilitate interactions between organisms. At the heart of the DOM cycle lie molecular-level relationships between the individual compounds in DOM and the members of the ocean microbiome that produce and consume them. In the past, these connections have eluded clear definition because of the sheer numerical complexity of both DOM molecules and microorganisms. Emerging tools in analytical chemistry, microbiology, and informatics are breaking down the barriers to a fuller appreciation of these connections. Here we highlight questions being addressed using recent meth- odological and technological developments in those fields and consider how these advances are trans- forming our understanding of some of the most important reactions of the marine carbon cycle. dissolved organic matter | marine microbes | cyberinfrastructure The global cycling of carbon supports life on Earth and and fundamental interactions have been necessarily affects the state of the biosphere within which humans oversimplified to yield a scientifically tractable reside. -

Biomineralization and Global Biogeochemical Cycles Philippe Van Cappellen Faculty of Geosciences, Utrecht University P.O
1122 Biomineralization and Global Biogeochemical Cycles Philippe Van Cappellen Faculty of Geosciences, Utrecht University P.O. Box 80021 3508 TA Utrecht, The Netherlands INTRODUCTION Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere- hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment. Biogeochemical cycles provide a general framework in which geochemists organize their knowledge and interpret their data. The cycle of a given element or substance maps out the rates of transformation in, and transport fluxes between, adjoining environmental reservoirs. The temporal and spatial scales of interest dictate the selection of reservoirs and processes included in the cycle. Typically, the need for a detailed representation of biological process rates and ecosystem structure decreases as the spatial and temporal time scales considered increase. Much progress has been made in the development of global-scale models of biogeochemical cycles. Although these models are based on fairly simple representations of the biosphere and hydrosphere, they account for the large-scale changes in the composition, redox state and biological productivity of the earth surface environment that have occurred over geological time. -

Distribution of Calcium Phosphate in the Exoskeleton of Larval Exeretonevra Angustifrons Hardy (Diptera: Xylophagidae)
Arthropod Structure & Development 34 (2005) 41–48 www.elsevier.com/locate/asd Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae) Bronwen W. Cribba,b,*, Ron Rascha, John Barrya, Christopher M. Palmerb,1 aCentre for Microscopy and Microanalysis, The University of Queensland, Brisbane, Qd 4072, Australia bDepartment of Zoology and Entomology, The University of Queensland, Brisbane, Qd 4072, Australia Received 28 July 2004; accepted 26 August 2004 Abstract Distribution and organisation of the mineral, amorphous calcium phosphate (ACP), has been investigated in the exoskeleton of the xylophagid fly larva Exeretonevra angustifrons Hardy. While head capsule and anal plate are smooth with a thin epicuticle, the epicuticle of the body is thicker and shows unusual micro-architecture comprised of minute hemispherical (dome-shaped) protrusions. Electron microprobe analysis and energy dispersive spectroscopy revealed heterogeneity of mineral elements across body cuticle and a concentration of ACP in the epicuticle, especially associated with the hemispherical structures. Further imaging and analysis showed the bulk of the ACP to be present in nano-sized granules. It is hypothesised that the specific distribution of ACP may enhance cuticular hardness or durability without reducing flexibility. q 2004 Elsevier Ltd. All rights reserved. Keywords: Insect; Cuticle; Integument; Hardening; Analytical electron microscopy; Electron microprobe 1. Introduction was distributed heterogeneously. Further investigation of the distribution of the mineral phase at the micron and Strengthening of biological structures through cuticular nanometre level is needed to discover where deposition is calcification is well developed in decapod crustaceans but it occurring and how this might affect exoskeletal organis- rarely occurs in insects, where it is poorly understood ation.