Appendix 1 Table A1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Original Research Article DOI - 10.26479/2017.0206.01 BIOLOGY of FEW BUTTERFLY SPECIES of AGRICULTURE ECOSYSTEMS of ARID REGIONS of KARNATAKA, INDIA Santhosh S
Santhosh & Basavarajappa RJLBPCS 2017 www.rjlbpcs.com Life Science Informatics Publications Original Research Article DOI - 10.26479/2017.0206.01 BIOLOGY OF FEW BUTTERFLY SPECIES OF AGRICULTURE ECOSYSTEMS OF ARID REGIONS OF KARNATAKA, INDIA Santhosh S. & S. Basavarajappa* Entomology Laboratory, DOS in Zoology, University of Mysore, Manasagangotri, Mysore-570 006, India ABSTRACT: Agriculture ecosystems have provided congenial habitat for various butterfly species. The Papilionidae and Nymphalidae family member’s most of their life cycle is depended on natural plant communities amidst agriculture ecosystems. To record few butterflies viz., Papilio polytes, Graphium agamemnon, Ariadne merione and Junonia hierta, agriculture ecosystems were selected randomly and visited frequently by adapting five-hundred-meter length line transects during 2014 to 2016. Study sites were visited during 0800 to 1700 hours and recorded the ovipositing behaviour of gravid female of these butterfly species by following standard methods. Eggs along with the host plant leaves / shoot / twigs were collected in a sterilized Petri dish and brought to the laboratory for further studies. Eggs were maintained under sterilized laboratory conditions till hatching. Newly hatched larvae were fed with their preferred host plants foliage and reared by following standard methods. P. polytes and G. agamemnon and A. merione and J. hierta developmental stages included egg, larva, pupa and adult and these stages have showed significant variation (F=21.35; P>0.01). Further, all the four species had four moults and five instars in their larval stage. However, including larval period, pupal duration was also varied considerably among these species. Further, overall life cycle completed in 43, 32.5 to 40, 21 to 30 and 21 to 29 days by P. -

Species Diversity and Community Structure of Butterfly in Urban Forest Fragments at Lucknow, India
Journal of Applied and Natural Science 10 (4): 1276-1280 (2018) ISSN : 0974-9411 (Print), 2231-5209 (Online) journals.ansfoundation.org Species diversity and community structure of butterfly in urban forest fragments at Lucknow, India Ashok Kumar* Article Info Department of Zoology, BSNVPG College (Lucknow University), Lucknow (U.P.), India DOI:10.31018/jans.v10i4.1908 Satyapal Singh Rana Received: September 26, 2018 Department of Zoology, S. M. P. Govt. Girls P.G. College, Meerut (U.P.), India Revised: November 18, 2018 Accepted: November 27, 2018 *Corresponding author. E-mail: ashokbsnv11gmail.com Abstract The survey was carried out between September 2015-August 2016 in five different locali- How to Cite ties in Lucknow like Bijli Pasi Quila, Smriti Upvan, Vanasthali Park, Butchery Ground and Kumar, A. and Rana, S.S. BSNVPG College Campus, Lucknow, 26.84’N latitude and 80.92’E longitude, is located at (2018). Species diversity an elevation of 126 meters above sea level and in the plain of northern India. Its location and community structure of is responsible for the diverse weather patterns and climate change. The butterfly in urban forest region has tropical dry equable climate having three main seasons; cold, hot and rainy fragments at Lucknow, season. Temperature of the city ranges from 23.8- 45.8°C in summer and 4.6-29.7°C in India. Journal of Applied winter. During the study, butterflies were collected mainly with the help of circular aerial and Natural Science, 10 net, which were then placed in killing jar. Killed butterflies were stored in the insect box by (4): 1276-1280 proper pinning them for identification. -

Euploea Species Are Unpalatable to Their Otherwise Enemies
An introduction to Crows of the World, the Euploeas (Lepidoptera : Danainae) – Peter Hendry The Euploea belong to the subfamily Danainae of the butterfly family Nymphalidae, although some authors place them in a family of their own, Danaidae. They are known as Crows, because of their mostly dark colouration, generally dark brown with some white markings. Some Crows have a blue or purplish sheen over part of their wings. With one endemic species on the Seychelles and another on the Mascarene Islands, in the Indian Ocean, the remainder of the genus ranges from India and South-east Asia (from Sikkim, Tibet and Afghanistan in the east) as far north as the Ryukyu Islands of southern Japan, throughout the Philippines and the Indonesian Archipelago, to New Guinea, the Bismarcks, Solomons, northern and eastern Australia, and the Islands associated with New Caledonia, the New Hebrides (Vanuatu), Fiji, Samoa, Tonga and as far eastwards as Niue, the Cook Islands and the Society Islands. (Parsons Fig. 1 1999) Fig.1. The larvae feed on plants in the Apocynaceae, Asclepiadaceae and Moraceae plant families, many of which are poisonous to most animals. Most of these plants extrude milky latex when cut. Caterpillars of the Common Crow, Euploea core corinna (Fig. 16) sever leaf veins prior to feeding on their latex-bearing host plants, which restricts the flow of latex at feeding sites (Clarke and Zalucki, 2001). This has been observed for other species and genera. Over 100 species of Lepidoptera, Coleoptera and Orthoptera cut veins on host plants (Helmus and Dussourd, 2003). By feeding on toxic plants the larvae, pupae and adults of some Euploea species are unpalatable to their otherwise enemies. -
New Host Records for Euploea Core Corinna (Macleay)
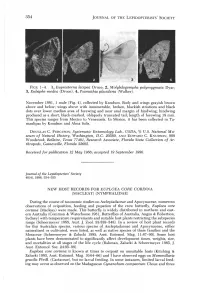
354 JOURNAL OF THE LEPIDOPTERISTS' SOCIETY FIGS. 1-4. 1, Euprosterna lacipea Druce; 2, Molybdogompha polymygmata Dyar; 3, Eubaphe medea (Druce); 4, Psamathia placidaria (Walker). November 1981, 1 male (Fig. 4), collected by Knudson. Body and wings grayish brown above and below; wings above with innumerable, broken, blackish striations and black dots over lower median area of forewing and near anal margin of hindwing; hindwing produced as a short, black-marked, obliquely truncated tail; length of forewing 18 mm. This species ranges from Mexico to Venezuela. In Mexico, it has been collected in Ta maulipas by Knudson and Alma Solis. DOUGLAS C. FERGUSON, Systematic Entomology Lab., USDA, % U.S. National Mu seum of Natural History, Washington, D.C. 20560, AND EDWARD C. KNUDSON, 808 Woodstock, Bellaire, Texas 77401, Research Associate, Florida State Collection of Ar thropods, Gainesville, Florida 32602. Received for publication 12 May 1986; accepted 19 September 1986. Journal of the Lepidopterists' Society 40(4), 1986, 354-355 NEW HOST RECORDS FOR EUPLOEA CORE CORINNA (MACLEA Y) (NYMPHALIDAE) During the course of taxonomic studies on Asclepiadaceae and Apocynaceae, numerous observations of oviposition, feeding and pupation of the crow butterfly, Euploea core corinna (Macleay) were made. This butterfly is widely distributed in northern and east ern Australia (Common & Waterhouse 1981, Butterflies of Australia, Angus & Robertson, Sydney) with temperature requirements and suitable host plants restricting the subspecies range (Scheermeyer 1985, Aust. J. Zoo I. 33:339-348). In a review of host plant records for this Australian species, various species of Asclepiadaceae and Apocynaceae, either naturalized or cultivated, were listed, as well as native species of these families and the Moraceae (Scheermeyer & Zalucki 1985, Aust. -

Observations on the Ecology of Euploea Core Corinna (Nymphalidae) with Special Reference to an Overwintering Population
Journal of the Lepidopterists' Society 35(2), 1981, 106-119 OBSERVATIONS ON THE ECOLOGY OF EUPLOEA CORE CORINNA (NYMPHALIDAE) WITH SPECIAL REFERENCE TO AN OVERWINTERING POPULATION R. L. KITCHING AND M. P. ZALUCKI School of Australian Environmental Studies, Griffith University, Nathan, QLD 4111, Australia ABSTRACT. A study of a sub-tropical overwintering aggregation of the common crow butterfly, Euploea core corinna (Nymphalidae: Danainae), has been made on the campus of Griffith University, Brisbane, Australia. Observations on the temporal and spatial phenology of the aggregation, together with the sex ratio, reproductive status, individual fat content and physical condition of butterflies within the aggregation are presented. The aggregation was restricted to the upper reaches of a gully on the campus between mid-May and late July. Physical conditions in the gully (temperature, humid ity and wind) were less severe than in adjacent areas. The butterflies in the gully were reproductively inactive. We suggest that such aggregations, apart from avoiding severe winter conditions, may also serve as mating concourses at the end of the winter season. The common crow butterfly, Euploea core (Cramer), is widely dis tributed in the Indo-Australian region and is represented in Australia by the subspecies, corinna (W. S. Macleay) (Fig. 1). Recent general ac counts of its life history are given by McCubbin (1971) and Common & Waterhouse (1972). These authors summarize information on food plants, phenology, behavior and morphology, principally collated from the observations of field naturalists. McCubbin (1971) alluded to the formation of overwintering aggregations of this species in shel tered coastal sites and offshore islands in the tropics and subtropics of northern and eastern Australia. -

Ring Roads and Urban Biodiversity: Distribution of Butterflies in Urban
www.nature.com/scientificreports OPEN Ring roads and urban biodiversity: distribution of butterfies in urban parks in Beijing city and Received: 1 June 2018 Accepted: 26 April 2019 correlations with other indicator Published: xx xx xxxx species Kong-Wah Sing1,2, Jiashan Luo3, Wenzhi Wang1,2,4,5, Narong Jaturas6, Masashi Soga7, Xianzhe Yang8, Hui Dong9 & John-James Wilson10,6,8 The capital of China, Beijing, has a history of more than 800 years of urbanization, representing a unique site for studies of urban ecology. Urbanization can severely impact butterfy communities, yet there have been no reports of the species richness and distribution of butterfies in urban parks in Beijing. Here, we conducted the frst butterfy survey in ten urban parks in Beijing and estimated butterfy species richness. Subsequently, we examined the distribution pattern of butterfy species and analyzed correlations between butterfy species richness with park variables (age, area and distance to city center), and richness of other bioindicator groups (birds and plants). We collected 587 individual butterfies belonging to 31 species from fve families; 74% of the species were considered cosmopolitan. The highest butterfy species richness and abundance was recorded at parks located at the edge of city and species richness was signifcantly positively correlated with distance from city center (p < 0.05). No signifcant correlations were detected between the species richness and park age, park area and other bioindicator groups (p > 0.05). Our study provides the frst data of butterfy species in urban Beijing, and serves as a baseline for further surveys and conservation eforts. China is a megadiverse country but is rapidly losing biodiversity as a consequence of socioeconomic development and expansion of urban land since the 1990s1,2. -

Patterns of Diversity, Abundance and Habitat Associations of Butterfly Communities in Heterogeneous Landscapes of the Department
International Journal of Biodiversity and Conservation Vol. 2(4), pp. 75-85, April 2010 Available online http://www.academicjournals.org/ijbc ISSN 2141-243X ©2010 Academic Journal Full Length Research Paper Patterns of diversity, abundance and habitat associations of butterfly communities in heterogeneous landscapes of the department of atomic energy (DAE) campus at Kalpakkam, South India T. Ramesh1, K. Jahir Hussain2, M. Selvanayagam1, K. K. Satpathy2* and M. V. R. Prasad2 1Loyola Institute of Frontier Energy (LIFE), Loyola College, Chennai- 600 034, India. 2Environmental and Industrial Safety Section, Safety Group, Indira Gandhi, Centre for Atomic Research, Kalpakkam- 603102, India. Accepted 28 January, 2010 The diversity of butterflies inhabiting the department of atomic energy campus at Kalpakkam was recorded through a modified line transect methodology by setting a permanent line transect of 300m and recoding all species of butterflies observed within a five meter distance around the observer. Five habitats within the campus viz., Garden, Scrub jungle, Riparian woods, Sandy area and Casuarina plantation (Monoculture) were evaluated for analysis of the association of the butterfly species with the habitat. A total of 1908 individuals representing 55 species were observed across the five habitat types. Out of these, members belonging to the family Nymphalidae was the most common with 20 species being recorded accounting for 36.3% of total species and 53.6% of total number of individuals collected. The maximum diversity and abundance was observed in the scrub jungle and garden area; these two habitats sharing 29 species among themselves. The species accumulation curve and rarefaction curves computed indicated the likelihood of encountering more number of species in the campus had inventory been more rigorous and extended. -

A Classification of Danaus Butterflies (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2005? 2005 1442 191212 Original Article CLASSIFICATION OF DANAUSD. A. S. SMITH ET AL. Zoological Journal of the Linnean Society, 2005, 144, 191–212. With 3 figures A classification of Danaus butterflies (Lepidoptera: Nymphalidae) based upon data from morphology and DNA DAVID A. S. SMITH FLS1*, GUGS LUSHAI FLS2 and JOHN A. ALLEN FLS2 1Natural History Museum, Eton College, Windsor SL4 6EW, UK 2Ecology & Biodiversity Division, School of Biological Sciences, University of Southampton SO16 7PX, UK Received September 2003; accepted for publication March 2005 Classification of the cosmopolitan butterfly genus Danaus (Nymphalidae: Danainae) is revised at subgeneric, specific and subspecific levels, combining for the first time mitochondrial and nuclear DNA sequence information with mor- phological data. Tree topologies based on the nuclear genome (allozymes, pheromone components, the morphology of all life history stages and nuclear DNA sequences), on the one hand, and mitochondrial DNA, on the other, are incon- gruent and challenge the current taxonomy of the genus. Although earlier classifications, based on adult morphology alone, are, in general, well supported by an analysis of total evidence, the mitochondrial phylogeny shows that the species D. chrysippus and its subgenus Anosia are deeply paraphyletic. Subspecies dorippus of D. chrysippus is the basal clade of the genus and is reinstated as the species D. dorippus. The former species D. plexaure is demoted to a subspecies of D. eresimus. The specific status of D. erippus, as distinct from D. plexippus, is tentatively supported. On the strength of the new data, division of the monophyletic genus Danaus s.l. -

Butterfly Diversity of the Central University of Tamil Nadu Campus in Thiruvarur, Tamil Nadu, India
ISSN 2230-7052 #176 ISSN 2230-7052 21 August 2019 No. 23, Feb 2017 No. 23, Feb 2017 XX XXXXXXX XXXX xxxxxxx 21 February 2017 XX XXXXXXX XXXX xxxxxxx 21 February 2017 Newsletter of the Newsletter of the Invertebrate Conservation & Information Network of South Asia (ICINSA) Invertebrate Conservation & Information Network of South Asia (ICINSA) Butterfly diversity of the Central University of Tamil Nadu Campus in Thiruvarur, Tamil Nadu, India The present study was an attempt to identified with standard reference books document the butterfly diversity of the such as Evans (1932), Wynter-Blyth (1957), Central University of Tamil Nadu (CUTN) Haribal (1992), Feltwell (2001), Kunte (2006) campus in Thiruvarur, Tamil Nadu, India. and Pajni et al. (2006). For common names CUTN (10.8190N & 79.6100E) is situated of butterflies, Wynter-Blyth (1957) and on both sides of Vettaru River, a major Varshney (1983) were followed. tributary of Cauvery River in the heart of the Cauvery Delta, 7km to the northwest The butterfly survey was carried out of Thiruvarur Town with 2.09 Km2 of land between 10.00h and 16.00h on days with in two revenue villages of Neelakudi and less than 50% cloud cover and moderate Nagakudi. The Thiruvarur district has light. Census routes were conceptualized a tropical climate, an average annual as transects with width of 12m (40ft). temperature of 28.50C, and an average Totally, six line transects were made within annual rainfall of 1,178mm. The campus the campus every month, totalling to 132 is situated in a riverine freshwater wetland transects in 11 months. -

Studies on Butterfly Diversity in Adichanalloor Village, Kollam
Journal of Entomology and Zoology Studies 2017; 5(5): 73-81 E-ISSN: 2320-7078 P-ISSN: 2349-6800 JEZS 2017; 5(5): 73-81 Studies on butterfly diversity in Adichanalloor © 2017 JEZS Village, Kollam District, Kerala Received: 11-07-2017 Accepted: 12-08-2017 Lekshmi Priya Lekshmi Priya, Varunprasath Krishnaraj, Janaranjini, Sutharsan and Department of Zoology, PSG Lakeshmanaswamy College of Arts and Science, Coimbatore, Tamil Nadu, India Abstract Varunprasath Krishnaraj The present investigation was carried out to study butterfly diversity in Adichanalloor Village, Kollam Department of Zoology, PSG district in Kerala, for the period of November 2016 to March 2017. Results showed that 79 species of College of Arts and Science, butterflies representing 5 major families were recorded. Family Nymphalidae showed the maximum Coimbatore, Tamil Nadu, India number of species followed by Lycanidae 13 species, Papilionidae 10 species, Pieridae 9 species and Hesperiidae 7 species. Among these families abundance of butterfly species in maximum in garden area Janaranjini (GI) with 21 species, followed by agrifield (GIII) (17 species), pond region (GV) (16 species), grassland Department of Zoology, PSG College of Arts and Science, (GII) (13 species) and shrubs and herbs (GIV) (12 species).Based on IUCN list, 49 species were Coimbatore, Tamil Nadu, India common(C), 27 species, uncommon (UC) and 3 species under rare category. According to monthly wise distribution of butterflies, maximum numbers of butterflies were recorded in November (32 species) Sutharsan followed by a December (21 species), January (12 species) and least in the month of March (8 species). Department of Zoology, PSG College of Arts and Science, Keywords: distribution, butterflies, Adichanalloor village, Kollam district, abundance. -

Sharma Ethno Ferns
CASE REPORT ZOOS' PRINT JOURNAL 18(2): 1003-1006 BUTTERFLIES OF SIRUVANI FORESTS OF WESTERN GHATS, WITH NOTES ON THEIR SEASONALITY P.R. Arun Salim Ali Centre for Ornithology and Natural History, Moongilpallam, Anaikatty, Coimbatore, Tamil Nadu 641108, India E-mail: [email protected] Abstract Study area The seasonal pattern in the abundance of butterflies The moist deciduous forests of Siruvani falling between 76o41'- belonging to the families, Papilionidae, Pieridae and 45' E and 10o56'-58' N in the Boluvampatti Reserve Forest located Nymphalidae was monitored during the period of in the foothills of Western Ghats about 35km west of Coimbatore, September 1994 to August 1996 in the moist deciduous Tamil Nadu, southern India was the selected study area. The forest of Siruvani, using the transect counting method. area receives good rainfall during both the north-east and south- Seventy-five species of butterflies belonging to 49 genera west monsoons. The mean annual rainfall of the area during the were recorded from the area during the study. Of these study period was 2092mm, much higher than that generally 53 were encountered along the transect. The present received by moist deciduous zones (Champion & Seth, 1968), paper also provides an account of their seasonality owing to the closeness of the study area to one of the core zone pattern. evergreen forests of the Nilgiri Biosphere Reserve. Keywords The transect selected for the study was about four kilometre off Butterflies, Lepidoptera, seasonal pattern, Siruvani, the forest check post at Sadivayal on the road towards the Water Nilgiri Biosphere Reserve, Western Ghats, southern Filtering plant of TWAD (Tamilnadu Water Supply and Drainage) India Board. -

Download Download
PLATINUM The Journal of Threatened Taxa (JoTT) is dedicated to building evidence for conservaton globally by publishing peer-reviewed artcles online OPEN ACCESS every month at a reasonably rapid rate at www.threatenedtaxa.org. All artcles published in JoTT are registered under Creatve Commons Atributon 4.0 Internatonal License unless otherwise mentoned. JoTT allows allows unrestricted use, reproducton, and distributon of artcles in any medium by providing adequate credit to the author(s) and the source of publicaton. Journal of Threatened Taxa Building evidence for conservaton globally www.threatenedtaxa.org ISSN 0974-7907 (Online) | ISSN 0974-7893 (Print) Communication Butterflies of the myristica swamp forests of Shendurney Wildlife Sanctuary in the southern Western Ghats, Kerala, India Prabhakaran Chandrika Sujitha, Gopal Prasad & Kalesh Sadasivan 26 February 2019 | Vol. 11 | No. 3 | Pages: 13320–13333 DOI: 10.11609/jot.4399.11.3.13320-13333 For Focus, Scope, Aims, Policies, and Guidelines visit htps://threatenedtaxa.org/index.php/JoTT/about/editorialPolicies#custom-0 For Artcle Submission Guidelines, visit htps://threatenedtaxa.org/index.php/JoTT/about/submissions#onlineSubmissions For Policies against Scientfc Misconduct, visit htps://threatenedtaxa.org/index.php/JoTT/about/editorialPolicies#custom-2 For reprints, contact <[email protected]> The opinions expressed by the authors do not refect the views of the Journal of Threatened Taxa, Wildlife Informaton Liaison Development Society, Zoo Outreach Organizaton, or any of the