Observations on the Ecology of Euploea Core Corinna (Nymphalidae) with Special Reference to an Overwintering Population
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

9 2013, No.1136
2013, No.1136 8 LAMPIRAN I PERATURAN MENTERI PERDAGANGAN REPUBLIK INDONESIA NOMOR 50/M-DAG/PER/9/2013 TENTANG KETENTUAN EKSPOR TUMBUHAN ALAM DAN SATWA LIAR YANG TIDAK DILINDUNGI UNDANG-UNDANG DAN TERMASUK DALAM DAFTAR CITES JENIS TUMBUHAN ALAM DAN SATWA LIAR YANG TIDAK DILINDUNGI UNDANG-UNDANG DAN TERMASUK DALAM DAFTAR CITES No. Pos Tarif/HS Uraian Barang Appendix I. Binatang Hidup Lainnya. - Binatang Menyusui (Mamalia) ex. 0106.11.00.00 Primata dari jenis : - Macaca fascicularis - Macaca nemestrina ex. 0106.19.00.00 Binatang menyusui lain-lain dari jenis: - Pteropus alecto - Pteropus vampyrus ex. 0106.20.00.00 Binatang melata (termasuk ular dan penyu) dari jenis: · Ular (Snakes) - Apodora papuana / Liasis olivaceus papuanus - Candoia aspera - Candoia carinata - Leiopython albertisi - Liasis fuscus - Liasis macklotti macklotti - Morelia amethistina - Morelia boeleni - Morelia spilota variegata - Naja sputatrix - Ophiophagus hannah - Ptyas mucosus - Python curtus - Python brongersmai - Python breitensteini - Python reticulates www.djpp.kemenkumham.go.id 9 2013, No.1136 No. Pos Tarif/HS Uraian Barang · Biawak (Monitors) - Varanus beccari - Varanus doreanus - Varanus dumerili - Varanus jobiensis - Varanus rudicollis - Varanus salvadori - Varanus salvator · Kura-Kura (Turtles) - Amyda cartilaginea - Calllagur borneoensis - Carettochelys insculpta - Chelodina mccordi - Cuora amboinensis - Heosemys spinosa - Indotestudo forsteni - Leucocephalon (Geoemyda) yuwonoi - Malayemys subtrijuga - Manouria emys - Notochelys platynota - Pelochelys bibroni -

Notes on Some Danaid Butterflies from Guangdong and Hainan (Lepidoptera: Rhopalocera)
NOTES ON SOME DANAID BUTTERFLIES FROM GUANGDONG AND HAINAN (LEPIDOPTERA: RHOPALOCERA) OKANOMasao and OKANOTetsuo In recent years, the knowledge on the. Danaid fauna of South’East Asia has bee・・em・・k・bly・d・qnced by M・RlsHITA(1977・1981).・.h・W・ve・・中・・e・till・erP・i・ some questions on the fauna ot the Tnainland of China apd .. the . island of Hainap. In this small paper we add a fqw novelties to several species of Danaidae from Guangdong (=Kwantung) and Hainan. Before goirig further we・wish to express our hearty thanks to Dr. T. SHiROzu for his kindness rendered in various ways; thanks are also’ due to-Mr. S. KolwAyA, Mr. S. KAMMuRI and T. SuzuKI for their assistance in securing materials, Ideopsis vulgaris contigua (TALBoT, 1939,) (PL 1,’ figs. 1, 5, 9) Danaus vulgaris: TALBoT, 1943: 147. Hainan. Dana”s vulgaris: TALBoT, 1947: 52. Hainan. Radena vulgaris contig. ua: MoRi$mTA, 1970: 68, .Hainan. ldeopsis vulgaris conti’ №浮=F MQRiSmTA, 1981: 504, 505. Hainan. Guangzhou, Guangdong:.2 9, August, 1980. This is the first record of the species from the continent of China and the northernmost record of the distribution of the species. M6reover, we have examined the following Hainan specimens. Tiaomengshan, Hainan: 1 6 1 9, December, 1980. Hainan: 2 6 2 9, January, 1981.・ Euploea sylvester hopei C. & R. FELDER,一 1865 (Pl. 2, figs. 1, 5, 6) Euploea (StictoPloea) harrisi bin’otata: JoicEy & TALBoT, 1924: 536. 2 6 2 9, Hainan.’ E”ploea (Stictoplbea) dufresne hopei: HuLsTAERT, 1931: 138. Hainan. Euploea sylvestor [sic]: SmR6zu, 1960: 118, text-fig. 146. -

National Recovery Plan for the Gove Crow Butterfly Euploea Alcathoe Enastri
NATIONAL RECOVERY PLAN FOR THE GOVE CROW BUTTERFLY Euploea alcathoe enastri Male Gove Crow Butterfly. Photo: © M.F. Braby 2 Prepared by Michael F. Braby, for the Department of Natural Resources, Environment and the Arts, Northern Territory Published by the Department of Natural Resources, Environment and the Arts, Northern Territory 2007 © Department of Natural Resources, Environment and the Arts, Northern Territory This work is copyright. It may be reproduced for study, research or training purposes subject to an acknowledgement of the sources but no commercial usage or sale. Requests and enquires concerning reproduction and rights should be addressed to: Invertebrate Scientist Department of Natural Resources, Environment and the Arts PO Box 496 PALMERSTON NT 0831 Note: This recovery plan sets out the actions necessary to stop the decline of, and support the recovery of, the Gove Crow Butterfly. The Australian Government is committed to acting in accordance with the plan and to implementing the plan as it applies to Commonwealth areas. This Territory approved recovery plan was prepared with financial support from the Australian Government and has been adopted as a national recovery plan under the provisions of the Commonwealth Environment Protection and Biodiversity Conservation Act 1999. The plan has been developed with the involvement and cooperation of a broad range of stakeholders, but individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge. -

Species Diversity and Community Structure of Butterfly in Urban Forest Fragments at Lucknow, India
Journal of Applied and Natural Science 10 (4): 1276-1280 (2018) ISSN : 0974-9411 (Print), 2231-5209 (Online) journals.ansfoundation.org Species diversity and community structure of butterfly in urban forest fragments at Lucknow, India Ashok Kumar* Article Info Department of Zoology, BSNVPG College (Lucknow University), Lucknow (U.P.), India DOI:10.31018/jans.v10i4.1908 Satyapal Singh Rana Received: September 26, 2018 Department of Zoology, S. M. P. Govt. Girls P.G. College, Meerut (U.P.), India Revised: November 18, 2018 Accepted: November 27, 2018 *Corresponding author. E-mail: ashokbsnv11gmail.com Abstract The survey was carried out between September 2015-August 2016 in five different locali- How to Cite ties in Lucknow like Bijli Pasi Quila, Smriti Upvan, Vanasthali Park, Butchery Ground and Kumar, A. and Rana, S.S. BSNVPG College Campus, Lucknow, 26.84’N latitude and 80.92’E longitude, is located at (2018). Species diversity an elevation of 126 meters above sea level and in the plain of northern India. Its location and community structure of is responsible for the diverse weather patterns and climate change. The butterfly in urban forest region has tropical dry equable climate having three main seasons; cold, hot and rainy fragments at Lucknow, season. Temperature of the city ranges from 23.8- 45.8°C in summer and 4.6-29.7°C in India. Journal of Applied winter. During the study, butterflies were collected mainly with the help of circular aerial and Natural Science, 10 net, which were then placed in killing jar. Killed butterflies were stored in the insect box by (4): 1276-1280 proper pinning them for identification. -

Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas
Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas Neal L. Evenhuis, Lucius G. Eldredge, Keith T. Arakaki, Darcy Oishi, Janis N. Garcia & William P. Haines Pacific Biological Survey, Bishop Museum, Honolulu, Hawaii 96817 Final Report November 2010 Prepared for: U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii Evenhuis et al. — Pagan Island Arthropod Survey 2 BISHOP MUSEUM The State Museum of Natural and Cultural History 1525 Bernice Street Honolulu, Hawai’i 96817–2704, USA Copyright© 2010 Bishop Museum All Rights Reserved Printed in the United States of America Contribution No. 2010-015 to the Pacific Biological Survey Evenhuis et al. — Pagan Island Arthropod Survey 3 TABLE OF CONTENTS Executive Summary ......................................................................................................... 5 Background ..................................................................................................................... 7 General History .............................................................................................................. 10 Previous Expeditions to Pagan Surveying Terrestrial Arthropods ................................ 12 Current Survey and List of Collecting Sites .................................................................. 18 Sampling Methods ......................................................................................................... 25 Survey Results .............................................................................................................. -
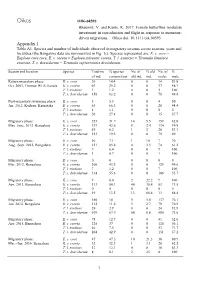
Appendix 1 Table A1
Oikos OIK-04593 Bhaumik, V. and Kunte, K. 2017. Female butterflies modulate investment in reproduction and flight in response to monsoon- driven migrations. – Oikos doi: 10.1111/oik.04593 Appendix 1 Table A1. Species and number of individuals observed in migratory swarms across seasons, years and localities (the Bangalore data are summarized in Fig. 1c). Species represented are: E. c. core = Euploea core core, E. s. coreta = Euploea sylvester coreta, T. l. exoticus = Tirumala limniace exoticus, T. s. dravidarum = Tirumala septentrionis dravidarum. Season and location Species Total no. % species No. of % old No. of % of ind. composition old ind. ind. males male Return migratory phase: E. c. core 26 10.4 0 0 14 53.8 Oct. 2001, Chinnar WLS, Kerala E. s. coreta 63 25.2 0 0 37 58.7 T. l. exoticus 3 1.2 0 0 3 100 T. s. dravidarum 158 63.2 0 0 78 49.4 Post-migratory swarming phase: E. c. core 5 5.3 0 0 4 80 Jan. 2012, Kodagu, Karnataka E. s. coreta 63 66.3 0 0 28 44.4 T. l. exoticus 1 1.1 0 0 0 0 T. s. dravidarum 26 27.4 0 0 15 57.7 Migratory phase: E. c. core 253 31.9 14 5.5 159 62.8 May–June, 2012, Bengaluru E. s. coreta 337 42.6 8 2.4 134 39.8 T. l. exoticus 49 6.2 1 2 26 53.1 T. s. dravidarum 153 19.3 0 0 75 49 Migratory phase: E. c. core 10 7.1 1 10 7 70 Aug.–Sept. -

Euploea Species Are Unpalatable to Their Otherwise Enemies
An introduction to Crows of the World, the Euploeas (Lepidoptera : Danainae) – Peter Hendry The Euploea belong to the subfamily Danainae of the butterfly family Nymphalidae, although some authors place them in a family of their own, Danaidae. They are known as Crows, because of their mostly dark colouration, generally dark brown with some white markings. Some Crows have a blue or purplish sheen over part of their wings. With one endemic species on the Seychelles and another on the Mascarene Islands, in the Indian Ocean, the remainder of the genus ranges from India and South-east Asia (from Sikkim, Tibet and Afghanistan in the east) as far north as the Ryukyu Islands of southern Japan, throughout the Philippines and the Indonesian Archipelago, to New Guinea, the Bismarcks, Solomons, northern and eastern Australia, and the Islands associated with New Caledonia, the New Hebrides (Vanuatu), Fiji, Samoa, Tonga and as far eastwards as Niue, the Cook Islands and the Society Islands. (Parsons Fig. 1 1999) Fig.1. The larvae feed on plants in the Apocynaceae, Asclepiadaceae and Moraceae plant families, many of which are poisonous to most animals. Most of these plants extrude milky latex when cut. Caterpillars of the Common Crow, Euploea core corinna (Fig. 16) sever leaf veins prior to feeding on their latex-bearing host plants, which restricts the flow of latex at feeding sites (Clarke and Zalucki, 2001). This has been observed for other species and genera. Over 100 species of Lepidoptera, Coleoptera and Orthoptera cut veins on host plants (Helmus and Dussourd, 2003). By feeding on toxic plants the larvae, pupae and adults of some Euploea species are unpalatable to their otherwise enemies. -
New Host Records for Euploea Core Corinna (Macleay)
354 JOURNAL OF THE LEPIDOPTERISTS' SOCIETY FIGS. 1-4. 1, Euprosterna lacipea Druce; 2, Molybdogompha polymygmata Dyar; 3, Eubaphe medea (Druce); 4, Psamathia placidaria (Walker). November 1981, 1 male (Fig. 4), collected by Knudson. Body and wings grayish brown above and below; wings above with innumerable, broken, blackish striations and black dots over lower median area of forewing and near anal margin of hindwing; hindwing produced as a short, black-marked, obliquely truncated tail; length of forewing 18 mm. This species ranges from Mexico to Venezuela. In Mexico, it has been collected in Ta maulipas by Knudson and Alma Solis. DOUGLAS C. FERGUSON, Systematic Entomology Lab., USDA, % U.S. National Mu seum of Natural History, Washington, D.C. 20560, AND EDWARD C. KNUDSON, 808 Woodstock, Bellaire, Texas 77401, Research Associate, Florida State Collection of Ar thropods, Gainesville, Florida 32602. Received for publication 12 May 1986; accepted 19 September 1986. Journal of the Lepidopterists' Society 40(4), 1986, 354-355 NEW HOST RECORDS FOR EUPLOEA CORE CORINNA (MACLEA Y) (NYMPHALIDAE) During the course of taxonomic studies on Asclepiadaceae and Apocynaceae, numerous observations of oviposition, feeding and pupation of the crow butterfly, Euploea core corinna (Macleay) were made. This butterfly is widely distributed in northern and east ern Australia (Common & Waterhouse 1981, Butterflies of Australia, Angus & Robertson, Sydney) with temperature requirements and suitable host plants restricting the subspecies range (Scheermeyer 1985, Aust. J. Zoo I. 33:339-348). In a review of host plant records for this Australian species, various species of Asclepiadaceae and Apocynaceae, either naturalized or cultivated, were listed, as well as native species of these families and the Moraceae (Scheermeyer & Zalucki 1985, Aust. -

STUDI KEANEKARAGAMAN HAYATI KUPU-KUPU (Sub Ordo Rhopalocera) DAN PERANAN EKOLOGISNYA DI AREA HUTAN LINDUNG KAKI GUNUNG PRAU KABUPATEN KENDAL JAWA TENGAH
STUDI KEANEKARAGAMAN HAYATI KUPU-KUPU (Sub Ordo Rhopalocera) DAN PERANAN EKOLOGISNYA DI AREA HUTAN LINDUNG KAKI GUNUNG PRAU KABUPATEN KENDAL JAWA TENGAH SKRIPSI Diajukan untuk Memenuhi Tugas dan Melengkapi Syarat Memperoleh Gelar Sarjana Pendidikan Ilmu Pendidikan Biologi Oleh: PURWOWIDODO NIM. 113811016 FAKULTAS ILMU TARBIYAH DAN KEGURUAN UNIVERSITAS ISLAM NEGERI WALISONGO SEMARANG 2015 i ii iii iv v ABSTRAK Judul : Studi Keanekaragaman Hayati Kupu-kupu (Sub Ordo Rhopalocera) dan Peranan Ekologisnya di Area Hutan Lindung Kaki Gunung Prau Kab. Kendal Jawa Tengah Penulis : Purwowidodo NIM : 113811016 Keanekaragaman kupu-kupu (Sub Ordo Rhopalocera) di Indonesia merupakan potensi kekayaan fauna yang belum banyak diketahui, termasuk di kawasan Hutan Lindung, Kaki Gunung Prau, Kabupaten Kendal, Jawa Tengah. Penelitian tentang keanekaragaman kupu-kupu dan peranannya secara ekologis telah dilakukan. Penelitian ini bertujuan untuk mengetahui tingkat keanekaragaman kupu-kupu dan peranan ekologisnya di area studi. Jenis penelitian kualitatif lapangan. Metode pengambilan data menggunakan purposive sampling dengan teknik transek sampel kuadrat (quadrat sampling transect) melalui tiga kali pengulangan. Analisis kuantitatif indeks biologi kupu-kupu menggunakan indeks keanekaragaman Shannon-Wiener, keseragaman atau kemerataan Pielou, dan dominansi Simpson. Keanekaragaman karakter jenis kupu-kupu, indeks perhitungan, dan peranan ekologisnya dianalisis secara deskriptif-kualitatif. Hasil penelitian diperoleh keanekaragaman jenis kupu-kupu sebanyak 34 spesies -

Arthropods Diversity As Ecological Indicators of Agricultural
Journal of Entomology and Zoology Studies 2020; 8(4): 1745-1753 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Arthropods diversity as ecological indicators of www.entomoljournal.com JEZS 2020; 8(4): 1745-1753 agricultural sustainability at la yaung taw, © 2020 JEZS Received: 05-05-2020 Naypyidaw union territory, Myanmar Accepted: 08-06-2020 Kyaw Lin Maung Biotechnology Research Department, Kyaw Lin Maung, Yin Yin Mon, Myat Phyu Khine, Khin Nyein Chan, Department of Research and Innovation, Ministry of Education (Science and Aye Phyoe, Aye Thandar Soe, Thae Yu Yu Han, Wah Wah Myo and Aye Technology), Kyauk-se, Myanmar Aye Khai Yin Yin Mon Biotechnology Research Department, Department of Research and Innovation, Ministry of Education (Science and Abstract Technology), Kyauk-se, Myanmar Arthropod diversity was considered as ecological indicators of sustainable agriculture and forest Myat Phyu Khine management. High-quality habitats have the relation with healthy ecosystem functioning. In this study, Biotechnology Research Department, we collected the 101 species of arthropods which consists of 40 species of butterflies, 19 species of flies, Department of Research and Innovation, 14 species of beetles, 10 species of grasshoppers, 7 species of wasps, 6 species of bugs, 3 species moths, Ministry of Education (Science and Technology), Kyauk-se, Myanmar 1 species of millipede and 1 species of centipede at la yaung taw, Naypyidaw union territory, Myanmar. Shannon-Wiener’s diversity indexes, Pielou’s Evenness Index (Equitability) and relative abundance in Khin Nyein Chan Biotechnology Research Department, arthropods were analyzed. Arthropod’s diversity index was observed as 1.717 while the evenness index Department of Research and Innovation, was 0.372. -

Euploea Alcathoe Enastri
Threatened Species of the Northern Territory GOVE CROW Euploea alcathoe enastri Conservation status Australia: Endangered Northern Territory: Near Threatened Photo: M Braby Description The Gove crow is a large, black-brown butterfly with variable white spots near the margins of the wings. The wingspan is about 70 mm. The male is velvet-black above and dark black-brown beneath. The female is paler chocolate-brown. Distribution The Gove Crow is a Northern Territory (NT) endemic, restricted to North-Eastern Arnhem Land. It was first discovered at Rocky Bay near Yirrkala in 1988 by G. Martin, and was subsequently recorded at three other locations, including Mosquito Creek Port Bradshaw, near Known locations of the Gove crow Mount Bonner, and the upper Goromuru River (Fenner 1991, 1992). Surveys carried out during Ecology 2006–2008 by Braby (2010) confirm that the Larval stages of E. a. enastri are found subspecies has a limited geographical range associated with several species of vines in the (extent of occurrence approximately 6,700 km2) Family Apocynaceae, and the preferred larval within which it is recorded from 21 sites food plant appears to be Parsonsia clustered within eleven locations or alboflavescens (Braby2009). This species subpopulations. Most sites comprise discrete occurs in patches of mixed paperbark tall open habitat patches that are small in area (<10 ha) forest with rainforest elements in the within which adults are localised and occur in understorey and rainforest edge (i.e. the relatively low abundance. ecotone between evergreen monsoon vine- forest and eucalypt/paperbark woodland). These Conservation reserves where reported: wet monsoon forest patches are always Nanydjaka Indigenous Protected Area. -

Yeasts Associated with Euploea Butterflies
Mycosphere 9(1): 149–154 (2018) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/9/1/4 Copyright © Guizhou Academy of Agricultural Sciences Yeasts associated with Euploea butterflies Lin WR1,2, Wang PH1*, Hsieh SY2, Tsai CH1, Hsiao SC1 1 Department of Life Science, Tunghai University, No. 1727, Sec. 4, Taiwan Boulevard, Taichung 40704, Taiwan. 2 Bioresource Collection and Research Center (BCRC), Food Industry Research and Development Institute (FIRDI), No. 331, Shih-Pin Road, Hsinchu 30062, Taiwan. Lin WR, Wang PH, Hsieh SY, Tsai CH, Hsiao SC 2018 – Yeasts associated with Euploea butterflies. Mycosphere 9(1), 149–154, Doi 10.5943/mycosphere/9/1/4 Abstract The yeasts were observed attached to the mouthparts, wings, and forelegs of migratory butterflies in Taiwan. Fifty-eight dominant yeast strains yeasts were isolated from 56 Euploea butterflies and identified by rDNA ITS and D1/D2 sequencing. The yeasts which associated with Euploea butterflies included ascomycetous yeasts, such as Aureobasidium sp., Candida chanthaburiensis, C. corydalis, Metschnikowia koreensis, Metschnikowia sp., and Debaryomyces hansenii; and basidiomycetous yeasts, such as Cryptococcus rajasthanensis, Dirkmeia churashimaensis, Filobasidium globisporum, Hannaella pagnoccae, Papiliotrema flavescens, Pseudozyma hubeiensis, P. tsukubaensis, Pseudozyma sp., Rhodotorula mucilaginosa, R. subericola, and Rhodosporidiobolus poonsookiae. The most common yeast, Candida corydalis, associated with 4 migratory Euploea species and seemed transmitted through nectar. When the butterflies feed on nectar, bask in the sun on the leaf, sip at the moisture on leaves, in puddles or wet sand and soil, they come into contact with yeasts. This is the first report about the yeast microbiome of migratory butterflies.