Prevalence of Enterohaemorrhagic Escherichia Coli O157:H7 In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

South·Africa in Transition
POLITICS OF HOPE AND TERROR: South ·Africa in Transition Report on Violence in South Africa by an American Friends Service Committee Study Team November 1992 The American Friends Service Committee's concern over Southern Africa has grown out of over 60 years of relationships since the first visit by a representative of the organization. In 1982 the AFSC Board of Directors approved the release of a full length book, Challenge and Hope, as a statement of its views on South Africa. Since 1977 the AFSC has had a national Southern Africa educational program in its Peace Education Division. AMERICAN FRIENDS SERVICE COMMITTEE 1501 Cherry Street Philadelphia, PA 19102 (215) 241-7000 AFSC REGIONAL OFFICES: Southeastern Region, Atlanta, Georgia 30303, 92 Piedmont Avenue, NE; Middle Atlantic Region, Baltimore, Maryland 21212, 4806 York Road; New England Region, Cambridge, Massachusetts 02140, 2161 Massachusetts Avenue; Great Lakes Region, Chicago, Illinois 60605, 59 E. Van Buren Street, Suite 1400; North Central Region, Des Moines, Iowa 50312, 4211 Grand Avenue; New York Metropolitan Region, New York, New York 10003, 15 Rutherford Place; Pacific Southwest Region, Pasadena, California 91103, 980 N. Fair Oaks Avenue; Pacific Mountain Region, San Francisco, California 94121,2160 Lake Street; Pacific Northwest Region, Seattle, Washington 98105, 814 N.E. 40th Street. CONTENTS II THE AFSC DELEGATION 1 PREFACE III POLITICS OF HOPE AND TERROR: South Africa in Transition 1 THE BASIC VIOLENCE 2 ANALYZING THE VIOLENCE 5 THE HIDDEN HAND 7 RETALIATION 9 POLICE INVESTIGATIONS 11 LESSONS FROM THE BOIPATONG MASSACRE 12 HOMELAND VIOLENCE IN CISKEI AND KWAZULU 13 HOMELAND LEADERS BUTHELEZI AND GQOZO 16 CONCLUSION 19 RECOMMENDATIONS 20 ACRONYMS 21 TEAM INTERVIEWS AND MEETINGS 22 THE AFSC DELEGATION TO SOUTH AFRICA The American Friends Service Committee's Board of Directors approved a proposal in June 1992 for a delegation to visit South Africa to study the escalating violence there. -

Local Economic Development Through Small Businesses in Dimbaza
LOCAL ECONOMIC DEVELOPMENT THROUGH SMALL BUSINESSES IN DIMBAZA By Sixolile Gantsho (212235222) Submitted in fulfilment of the requirements for the Master of Arts degree (MA): Economics Research In the Faculty of Business and Economic Sciences Of the Nelson Mandela University August 2019 Supervisor: Professor Ronney Ncwadi ABSTRACT This study was conducted in Dimbaza, a small town outside of King Williams Town. Dimbaza was created as a resettlement township during Apartheid in 1967. The study investigated local economic development through small businesses. The study described the state of the small business sector, it presented the contribution this sector has towards employment creation and income generation. Furthermore, it investigated the challenges that the small business sector in Dimbaza faces and the level of responsiveness from the local authorities in Buffalo City Metropolitan Municipality which Dimbaza falls under. The study was conducted using a survey questionnaire which was administered to small business entrepreneurs. Furthermore, a focus group with small business owners and semi-structured interviews were conducted with officials from the Buffalo City Metropolitan Municipality in the Business Development and Local Economic Development Directorate. The findings of the study suggest that Dimbaza has a large informal economy which has of survivalist entrepreneurs with small and micro businesses. It is evident from the study that the small business sector in Dimbaza is facing the following challenges: 1) No access to funding opportunities, 2) a lack of infrastructure, 3) lack of proper running water and electricity and 4) lack of economic development programmes from the municipality. There is a dire need for the intervention of local authorities in the small business sector in Dimbaza. -

Buffalo City Municipality State of Energy Report Table of Contents
BUFFALO CITY MUNICIPALITY SSSTTTAAATTTEEE OOOFFF EEENNNEEERRRGGGYYY RRREEEPPPOOORRRTTT J28015 September 2008 EXECUTIVE SUMMARY The Importance of Sustainable Energy to BCM South African cities are key players in facilitating national sustainable energy policy and legislative objectives. The 15 largest cities in South Africa take up 3% of the country’s surface area, and yet they are responsible for 40% of the country’s energy consumption. This means that cities must play a major role in facilitating the achievement of national sustainable energy targets (for example the national target of 12% energy efficiency by 2014). Buffalo City, being among the nine largest cities in South Africa, and the second largest in the Eastern Cape, must ensure that it participates in, and takes responsibility for, energy issues affecting both its own population, and that of the country as a whole. Issues associated with the availability and use of energy in South Africa and the Eastern Cape are more pressing than ever before. Some of the more urgent considerations are related to the following: Climate Change: Scientific evidence shows without doubt that the earth’s atmosphere has been heating up for the past century (global warming), and that this heating is due to greenhouse gas emissions from the burning of the fossil fuels (such as coal and oil products) from which we derive our energy. Some impacts of climate change that scientists have predicted will affect Southern Africa (including BCM) are: • More disasters related to severe weather events; • Longer and drier dry periods, leading to drought; • More runaway fires; • More intense flooding; • Sea-level rise; • Threats to food security and human health; • Loss of biodiversity; • Water supply problems; and • Related economic impacts Climate change is already causing negative impacts on people and ecosystems in South Africa. -

Case Study of King William's Town
Water Supply Services Model: Case Study of King William's Town Report to the Water Research Commission by Palmer Development Group WRC Report No KV110/98 Disclaimer This report emanates from a project financed by the Water Research Commission (WRC) and is approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the WRC or the members of the project steering committee, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. Vry waring Hierdie verslag spruit voort uit 'n navorsingsprojek wat dour die Waternavorsingskommissie (WNK) gefinansier is en goedgekeur is vir publikasie. Gocdkcuring beteken nie noodwendig dat die inhoud die sicning en bclcid van die WNK of die lede van die projek-loodskoinitee weerspieel nie, of dat melding van handelsname of -ware deur die WNK vir gebruik goedgekeur of aanbeveel word nie. WATER SUPPLY SERVICES MODEL : CASE STUDY OF KING WILLIAM'S TOWN Report on application of the WSSM to the King William's Town TLC PALMER DEVELOPMENT GROUP WRC Report No KV110/98 ISBN 1 86845 407 X ISBN SET 1 86845 408 8 ACKNOWLEDGEMENTS We wish to extend our thanks to the officials and councillors of the King William's Town TLC for giving us access to the information necessary to conduct this study. We would in particular like to thank the Town Engineer Chris Hetem, the Treasurer Gideon Thiart, Hans Schluter of the Planning Department and Trevor Belser of the Department of Water Affairs and Forestry for their time and support during the course of the study. -

Directory of Organisations and Resources for People with Disabilities in South Africa
DISABILITY ALL SORTS A DIRECTORY OF ORGANISATIONS AND RESOURCES FOR PEOPLE WITH DISABILITIES IN SOUTH AFRICA University of South Africa CONTENTS FOREWORD ADVOCACY — ALL DISABILITIES ADVOCACY — DISABILITY-SPECIFIC ACCOMMODATION (SUGGESTIONS FOR WORK AND EDUCATION) AIRLINES THAT ACCOMMODATE WHEELCHAIRS ARTS ASSISTANCE AND THERAPY DOGS ASSISTIVE DEVICES FOR HIRE ASSISTIVE DEVICES FOR PURCHASE ASSISTIVE DEVICES — MAIL ORDER ASSISTIVE DEVICES — REPAIRS ASSISTIVE DEVICES — RESOURCE AND INFORMATION CENTRE BACK SUPPORT BOOKS, DISABILITY GUIDES AND INFORMATION RESOURCES BRAILLE AND AUDIO PRODUCTION BREATHING SUPPORT BUILDING OF RAMPS BURSARIES CAREGIVERS AND NURSES CAREGIVERS AND NURSES — EASTERN CAPE CAREGIVERS AND NURSES — FREE STATE CAREGIVERS AND NURSES — GAUTENG CAREGIVERS AND NURSES — KWAZULU-NATAL CAREGIVERS AND NURSES — LIMPOPO CAREGIVERS AND NURSES — MPUMALANGA CAREGIVERS AND NURSES — NORTHERN CAPE CAREGIVERS AND NURSES — NORTH WEST CAREGIVERS AND NURSES — WESTERN CAPE CHARITY/GIFT SHOPS COMMUNITY SERVICE ORGANISATIONS COMPENSATION FOR WORKPLACE INJURIES COMPLEMENTARY THERAPIES CONVERSION OF VEHICLES COUNSELLING CRÈCHES DAY CARE CENTRES — EASTERN CAPE DAY CARE CENTRES — FREE STATE 1 DAY CARE CENTRES — GAUTENG DAY CARE CENTRES — KWAZULU-NATAL DAY CARE CENTRES — LIMPOPO DAY CARE CENTRES — MPUMALANGA DAY CARE CENTRES — WESTERN CAPE DISABILITY EQUITY CONSULTANTS DISABILITY MAGAZINES AND NEWSLETTERS DISABILITY MANAGEMENT DISABILITY SENSITISATION PROJECTS DISABILITY STUDIES DRIVING SCHOOLS E-LEARNING END-OF-LIFE DETERMINATION ENTREPRENEURIAL -

Draft Built Environment Performance Plan 2017/2018
DRAFT BUILT ENVIRONMENT PERFORMANCE PLAN 2017/2018 Draft 1 TABLE OF CONTENTS INTRODUCTION……………………………………………………............................................................................6 PROFILE OF THE BUFFALO CITY METROPOLITAN MUNICIPALITY ............................................................................... 6 SECTION A ........................................................................................................................................................................ 9 A.1. BEPP IN RELATION TO OTHER STATUTORY PLANS .............................................................................................. 10 A.1.1. BCMM Documents: .......................................................................................................................................... 11 A.1.2. National and Provincial Documents: .............................................................................................................. 11 A.1.3. Aligning the BEPP with IDP, MGDS, BCMM SDF and Budget ................................................................. 12 A.1.4. Confirmation of BEPP Adoption by Council ..................................................... Error! Bookmark not defined. SECTION B : SPATIAL PLANNING &PROJECT PRIORITISATION ...................................................................................... 14 B.1. SPATIAL TARGETING ............................................................................................................................... 14 (a) The National Development Plan -

Buffalo City Metro Municipality Socio Economic Review and Outlook, 2017
BUFFALO CITY METRO MUNICIPALITY SOCIO ECONOMIC REVIEW AND OUTLOOK, 2017 Buffalo City Metro Municipality Socio-Economic Review and Outlook 2017 Published by ECSECC Postnet Vincent, P/Bag X9063, Suite No 302, Vincent 5247 www.ecsecc.org © 2017 Eastern Cape Socio Economic Consultative Council First published April 2017 Some rights reserved. Please acknowledge the author and publisher if utilising this publication or any material contained herein. Reproduction of material in this publication for resale or other commercial purposes is prohibited without written permission from ECSECC. Buffalo City Metro Municipality Socio-Economic Review and Outlook 2017 Foreword ECSECC was founded in July 1995 as an institutional mechanism for partnership between government, business, labour and the NGO sector to address underdevelopment and poverty in the Eastern Cape. The local government sector and the higher education sector joined ECSECC in 2003. ECSECC’s mandate of stakeholder co-ordination and multi-stakeholder policy making stems from the realization that Government cannot defeat poverty, unemployment and inequality on its own, but needs to build deliberate and active partnerships to achieve prioritized development outcomes. ECSECCs main partners are: the shareholder, the Office of the Premier; national, provincial and local government; organised business and industry; organised labour; higher education; and the organised NGO sectors that make up the board, SALGA and municipalities. One of ECSECCs goals is to be a socio-economic knowledge hub for the Eastern Cape Province. We seek to actively serve the Eastern Cape’s needs to socio-economic data and analysis. As part of this ECSECC regularly issues statistical and research based publications. Publications, reports and data can be found on ECSECCs website www.ecsecc.org. -

Profile: Buffalo City
2 PROFILE: BUFFALO CITY PROFILE: BUFFALO CITY 3 CONTENT 1. Executive Summary…………………………………………………………………….3 2. Introduction: Brief Overview ............................................................................. 6 2.1 Location ..................................................................................................................................... 6 2.2 Historical Pesperctive ................................................................................................................ 6 2.3 Spatial Status ............................................................................................................................. 7 3. Social Development Profile ............................................................................... 8 3.1 Key Social Demographics ........................................................................................ 8 3.1.1 Population ............................................................................................................. 8 3.1.2 Race, Gender and Age ........................................................................................ 10 3.1.3 Households ......................................................................................................... 10 3.2 Health Profile .......................................................................................................... 11 3.3 COVID-19 .............................................................................................................. 11 3.4 Poverty Dimensions .............................................................................................. -

Eastern Cape
TELEPHONE COST CENTRE BRANCH SERVICE STREET ADDRESS FAX NUMBER NUMBER AREA MANAGER 259 Govan Mbeki Ave 041-508 4260 0866 882 260 60053 Addo Renewals MAIN ST 042-2330336 042 2330336 60502 ALGOA PARK Renewals 3 St Leonards Rd, Algoa Park 041-4522203 041-4527413 63602 CENTRAHIL Renewals 3 Rink St, Central 041-5858345 041-5855701 64853 DESPATCH Full c\o Rabie & Main Str, Despatch 041-9335106 041-9332659 66002 EMERALD HILL Renewals Rhodes Street, Mt Pleasant 041-3673050 041-3673336 66864 GELVANDALE Full Gail Road,Gelvandale 041-4522109 041-4561161 78912 GREENACRES Full Ring Road, Greenacres 041-3630765 041-3632702 67857 Hankey Renewals Hoof Straat, CENTRAL 042-2840239 042 2840210 69054 Humansdorp Renewals 18 DU PLESSIS ST 042-2951063 042 2911050 69101 HUMEWOOD Renewals 5B Humeway Shopping Centre, Humewood 041-5840634 041-5840611 68825 HUNTER'S RETREAT Renewals Kwik Spar Old Cape Rd 041-3606071 041-3605131 69503 Jeffreysbay Renewals 9 DE REIGER ST 042-2931240 042 2932352 69552 Joubertina Renewals VAN RIEBEECK ST 042-2732233 042 2732344 70501 Kirkwood Renewals VOORTREKKER RD 042-2300444 042 2301728 71609 KORSTEN Renewals 20 Essex Street, Korsten 041-4511362 041-4532582 69834 KWADWESI Renewals 32 Ziyabuya Shopping Centre, Kwadwesi 041-4850515 041-4852321 72022 KWANOBUHLE Renewals Jabavu Street, Kwanobuhle, Uitenhage 041-9771504 041-9771504 73098 LINTON GRANGE Renewals 529 Cape Road, Linton Grange 041-3607808 041-3605145 76936 NEWTONPARK Full Fifth Avenue, Newton Park 041-3641067 041-3656290 77358 NORTH END Full 3 Parkin Street, North End 041-4844553 -

The Status of Traditional Horse Racing in the Eastern Cape
THR Cover FA 9/10/13 10:49 AM Page 1 The Status of Traditional Horse Racing in the Eastern Cape www.ussa.org.za www.ru.ac.za THR Intro - Chp 3 FA 9/10/13 10:40 AM Page 1 The Status of Traditional Horse Racing in the Eastern Cape ECGBB – 12/13 – RFQ – 10 Commissioned by Eastern Cape Gambling and Betting Board (ECGBB) Rhodes University, Grahamstown, Eastern Cape, was awarded incidental thereto, contemplated in the Act and to advise the a tender called for by the Eastern Cape Gambling and Betting Member of the Executive Council of the Province for Economic Board (ECGBB) (BID NUMBER: ECGBB - 12/13 RFQ-10) to Affairs and Tourism (DEAT) with regard to gambling matters undertake research which would determine the status of and to exercise certain further powers contemplated in the traditional horse racing (THR) in the Eastern Cape. Act. The ECGBB was established by section 3 of the Gambling Rhodes University, established in 1904, is located in and Betting Act, 1997 (Act No 5 of 1997, Eastern Cape, as Grahamstown in the Eastern Cape province of South Africa. amended). The mandate of the ECGBB is to oversee all Rhodes is a publicly funded University with a well established gambling and betting activities in the Province and matters research track record and a reputation for academic excellence. Rhodes University Research Team: Project Manager: Ms Jaine Roberts, Director: Research Principal Investigator: Ms Michelle Griffith Senior Researcher: Mr Craig Paterson, Doctoral Candidate in History Administrator: Ms Thumeka Mantolo, Research Officer, Research Office Eastern Cape Gambling & Betting Board: Marketing & Research Specialist: Mr Monde Duma Cover picture: People dance and sing while leading horses down to race. -

Accredited COVID-19 Vaccination Sites Eastern Cape
Accredited COVID-19 Vaccination Sites Eastern Cape Permit Primary Name Address Number 202103960 Fonteine Park Apteek 115 Da Gama Rd, Ferreira Town, Jeffreys Bay Sarah Baartman DM Eastern Cape 202103949 Mqhele Clinic Mpakama, Mqhele Location Elliotdale Amathole DM Eastern Cape 202103754 Masincedane Clinic Lukhanyisweni Location Amathole DM Eastern Cape 202103840 ISUZU STRUANWAY OCCUPATIONAL N Mandela Bay MM CLINIC Eastern Cape 202103753 Glenmore Clinic Glenmore Clinic Glenmore Location Peddie Amathole DM Eastern Cape 202103725 Pricesdale Clinic Mbekweni Village Whittlesea C Hani DM Eastern Cape 202103724 Lubisi Clinic Po Southeville A/A Lubisi C Hani DM Eastern Cape 202103721 Eureka Clinic 1228 Angelier Street 9744 Joe Gqabi DM Eastern Cape 202103586 Bengu Clinic Bengu Lady Frere (Emalahleni) C Hani DM Eastern Cape 202103588 ISUZU PENSIONERS KEMPSTON ROAD N Mandela Bay MM Eastern Cape 202103584 Mhlanga Clinic Mlhaya Cliwe St Augustine Jss C Hani DM Eastern Cape 202103658 Westering Medicross 541 Cape Road, Linton Grange, Port Elizabeth N Mandela Bay MM Eastern Cape Updated: 30/06/2021 202103581 Tsengiwe Clinic Next To Tsengiwe J.P.S C Hani DM Eastern Cape 202103571 Askeaton Clinic Next To B.B. Mdledle J.S.School Askeaton C Hani DM Eastern Cape 202103433 Qitsi Clinic Mdibaniso Aa, Qitsi Cofimvaba C Hani DM Eastern Cape 202103227 Punzana Clinic Tildin Lp School Tildin Location Peddie Amathole DM Eastern Cape 202103186 Nkanga Clinic Nkanga Clinic Nkanga Aa Libode O Tambo DM Eastern Cape 202103214 Lotana Clinic Next To Lotana Clinic Lotana -
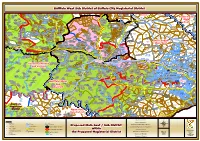
EC Bcsub 032018 Bufffaolwest.Pdf
!C !C^ ñ!.!C !C $ !C^ ^ ^ !C !C !C!C !C !C !C ^ ^ !C !C ^ !C !C !C !C !C ^ !C ñ !C !C !C !C !C !C ^ !C !C ^ !C $ !C ^ !C !C !C !C !C !C ^ !C ^ !C !C !C ñ !C !C !C !C !C !C !C !C !C !. !C ^ ^ !C ñ !C !C !C !C !C !C !C ^$ !C ^ !C !C !C ñ !C !C !C ñ!C ^ ñ!C !. !C !C !C ^ !C ^ !C ^ !C ^ !C !C !C !C !C !C !C !C ^ !C ñ !C !C !C !C !C ñ^ !C !C ñ !C !C !C !C !C !C !C !C !C !C !C !C ñ !C !C ^ ^ !C !C !C !. !C ñ ^!C ^ !C !C !C ñ ^ ^ !C !C $ ^ $!C ^ !C !C !C !C !C !C !C !C !C !C !C !C !. !C !.^ $ ñ !C !C !C !C ^ !C !C !C $ !C ^ !C $ !C !C !C $ ñ !. !C !C !C !C !C !C !. ^ ñ!C ^ ^ !C $!. ^ !C !C !C !C !C !C !C !C !C !C !C !C !C !C !C !. !C !C !C !C ^ !C !C !. !C !C !C ñ !C ^ñ !C !C ñ !C !C !. ^ !C !C !C !C !C !C !C ^ !Cñ ^$ ^ !C ñ !C !. ñ!C ^ !C !. !C !C ^ ^ ñ !. !C !C $^ ^ñ ^ !C ^ ñ ^ ^ !C !C !C !C !C !C !C ^ !C !C !C !C !C !C !C !C !. !C ^ !C $ !. ñ!C !C !C ^ !C. ñ^ !C !C !C !C !C !C !C !C $!C ^!. !. !. !C !C !. ^ !C !C ^ !C !C ^ !C ñ!C !C !. !C $^ !C !C !C !C !C !C !.