A Comprehensive Guide to Method Development Using Triple Quadrupole ICP-MS a Comprehensive Guide to Method Development Using Triple Quadrupole ICP-MS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Circular of the Bureau of Standards No. 562: Bibliography of Research on Deuterium and Tritium Compounds 1945 and 1952
NBS CIRCULAR 562 Bibliography of Research on Deuterium and Tritium Compounds 1945 to 1952 UNITED STATES DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS PERIODICALS OF THE NATIONAL BUREAU OF STANDARDS (Published monthly) The National Bureau of Standards is engaged in fundamental and applied research in physics, chemistry, mathematics, and engineering. Projects are conducted in fifteen fields: electricity and electronics, optics and metrology, heat and power, atomic and radiation physics, chemistry, mechanics, organic and fibrous materials, metallurgy, mineral products, building technology, applied mathematics, data process¬ ing systems, cryogenic engineering, radio propagation, and radio standards. The Bureau has custody of the national standards of measurement and conducts research leading to the improvement of scientific and engineering standards and of techniques and methods of measurement. Testing methods and in¬ struments are developed; physical constants and properties of materials are determined; and technical processes are investigated. Journal of Research The Journal presents research papers by authorities in the specialized fields of physics, mathematics, chemistry, and engineering. Complete details of the work are presented, including laboratory data, experimental procedures, and theoretical and mathematical analyses. Annual subscription: domestic, $4.00; 25 cents additional for foreign mailing. Technical News Bulletin Summaries of current research at the National Bureau of Standards are published each month in the Technical News Bulletin. The articles are brief, with emphasis on the results of research, chosen on the basis of their scientific or technologic importance. Lists of all Bureau publications during the preceding month are given, including Research Papers, Handbooks, Applied Mathematics Series, Building Mate¬ rials and Structures Reports, Miscellaneous Publications, and Circulars. -

The Radiochemistry of Tungsten
National Academy of Sciences !National Research Council NUCLEAR SCIENCE SERIES The Radiochemistry of Tungsten — ...—- L. F. C URTISS,Chairman ROBLEY D. EVANS, Vice Chairman NationalBureau ofStandards MassachusettsInstituteofTechnology J.A. DeJUREN, Secretary WestinghouseElectricCorporation C. J.BORKOWSKI J.W. IRVINE,JR. Oak RidgeNationalLaboratory MassachusettsI&tituteofTechnology ROBERT G. COCHRAN E. D. KLEMA Texas Agriculturaland Mechanical NorthwesternUniversity College W. WAYNE MEINKE SAMUEL EPSTEIN UniversityofMichigan CaliforniaInstituteofTechnology J.J.NICKSON Memorial Hospital,New York U. FANO NationalBureau ofStandards ROBERT L. PLATZMAN Laboratoirede Chimie Physique HERBERT GOLDSTEIN NuclearDevelopmentCorporationof D. M. VAN PATTER America BartolResearch Foundation LIAISON MEMBERS PAUL C. AEBERSOLD CHARLES K. REED Atomic Energy Commission U. S.Air Force J.HOWARD McMILLEN WILLIAM E. WRIGHT NationalScienceFoundation OfficeofNavalResearch SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chai~man HAROLD KIRBY UniversityofMichigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE FloridaStateUniversity Oak RidgeNationalLaboratory GEORGE A. COWAN JULIAN NIELSEN Los Alamos ScientificLaboratory HanfordLaboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG UniversityofWashington Argonne NationalLaboratory JEROME HUDIS PETER C. STEVENSON BrookhavenNationalLaboratory UniversityofCalifornia(Livermore) EARL HYDE LEO YAFFE UniversityofC slifornia(Berkeley) McGillUniversity CONSULTANTS NATHAN BALLOU JAMES DeVOE NavalRadiologicalDefenseLaboratory -
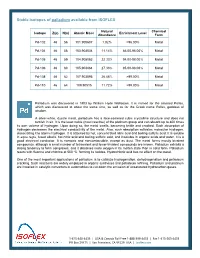
Stable Isotopes of Palladium Available from ISOFLEX
Stable isotopes of palladium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Pd-102 46 56 101.905607 1.02% >96.00% Metal Pd-104 46 58 103.904034 11.14% 86.00-98.00% Metal Pd-105 46 59 104.905083 22.33% 94.00-98.00% Metal Pd-106 46 60 105.903484 27.33% 95.00-98.00% Metal Pd-108 46 62 107.903895 26.46% >99.00% Metal Pd-110 46 64 109.90515 11.72% >99.00% Metal Palladium was discovered in 1803 by William Hyde Wollaston. It is named for the asteroid Pallas, which was discovered at about the same time, as well as for the Greek name Pallas, goddess of wisdom. A silver-white, ductile metal, palladium has a face-centered cubic crystalline structure and does not tarnish in air. It is the least noble (most reactive) of the platinum group and can absorb up to 800 times its own volume of hydrogen. Upon doing so, the metal swells, becoming brittle and cracked. Such absorption of hydrogen decreases the electrical conductivity of the metal. Also, such absorption activates molecular hydrogen, dissociating it to atomic hydrogen. It is attacked by hot, concentrated nitric acid and boiling sulfuric acid. It is soluble in aqua regia, fused alkalis, hot nitric acid and boiling sulfuric acid, and insoluble in organic acids and water. It is a good electrical conductor. It is nontoxic and noncombustible, except as dust. The metal forms mostly bivalent compounds, although a small number of tetravalent and fewer trivalent compounds are known. -

Creation and Nuclear Reaction Studies Sorption Behaviour of Short
PL9601122 PL9601121 High-Spin Nuclear Target of 178m2Hf: Creation and Nuclear Reaction Studies Yu. Ts. Oganesian1, S.A. Karamian1, Yu. P. Gangrsky1, B. Gorski1, B.N. Markov1, Z. Szeglowski2, Ch. Briangon3, O. Constantinescu3, M. Hussonnois3, J. Pinard3, R. Kulessa4, H.J. Wollersheim4, G. Graw5, J. de Boer5, G. Huber6 and H.V. Muradian7 XFLNR, JINR Dubna, Russia; 2H. Niewodniczanski Institute Nuclear Physics, Krakow, Poland; 3CSNSM, IPN, Orsay, France; 4GSI, Darmstadt, Germany; 6Miinchen University, Germany; 6Mainz University, Germany; 7Kurchatov Institute, Moscow, Russia. Investigations of the hafnium-178 isomers are a new scientific direction promising the devel- opment of a fundamental knowledge both in the field of the nuclear structure and of nuclear reactions. The completed experiments give grounds for hope of obtaining data on the electro- magnetic moments, on the mean radius and the deformation of the 178Hf nucleus in the state 16+, on the wave function structure of this state, as well as to study the influence of the target high spin on the differential cross sections of nuclear reactions, to find and investigate neutron resonances with a high spin, to obtain direct information on the density of the levels in the earlier inaccessible region of the spin and of the excitation energy, to measure directly the parameters of a giant dipole resonance based on the high spin state and to clarify in detail the role of the structure hindrances in nuclear reactions. Sorption Behaviour of Short-Lived W and Hf Isotopes on Ion Exchangers from HC1/HF Solutions in Fast On-Line Experiments D. Schumann1, R. Dressier1, S. Fischer1, St. -

Cosmogenic Production As a Background in Searching For
Cosmogenic Production as a Background in Searching for Rare Physics Processes D.-M. Mei a , Z.-B. Yin a,b,c,1, S. R. Elliott d aDepartment of Physics, The University of South Dakota, Vermillion, South Dakota 57069 bInstitute of Particle Physics, Huazhong Normal University, Wuhan 430079, China cKey Laboratory of Quark & Lepton Physics (Huazhong Normal University), Ministry of Education, China dLos Alamos National Laboratory, Los Alamos, New Mexico 87545 Abstract We revisit calculations of the cosmogenic production rates for several long-lived iso- topes that are potential sources of background in searching for rare physics processes such as the detection of dark matter and neutrinoless double-beta decay. Using up- dated cosmic-ray neutron flux measurements, we use TALYS 1.0 to investigate the cosmogenic activation of stable isotopes of several detector targets and find that the cosmogenic isotopes produced inside the target materials and cryostat can result in large backgrounds for dark matter searches and neutrinoless double-beta decay. We use previously published low-background HPGe data to constrain the production of 3H on the surface and the upper limit is consistent with our calculation. We note that cosmogenic production of several isotopes in various targets can generate po- arXiv:0903.2273v1 [nucl-ex] 12 Mar 2009 tential backgrounds for dark matter detection and neutrinoless double-beta decay with a massive detector, thus great care should be taken to limit and/or deal with the cosmogenic activation of the targets. Key words: Cosmogenic activation, Dark matter detection, Double-beta decay PACS: 13.85.Tp, 23.40-s, 25.40.Sc, 28.41.Qb, 95.35.+d, 29.40.Wk Email address: [email protected] (D.-M. -

(12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden Et Al
USOO8625731B2 (12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden et al. (45) Date of Patent: Jan. 7, 2014 (54) COMPACT NEUTRONGENERATOR FOR (51) Int. Cl. MEDICAL AND COMMERCIAL SOTOPE G2G 4/02 (2006.01) PRODUCTION, FISSION PRODUCT G2G 4/00 (2006.01) PURIFICATION AND CONTROLLED (52) U.S. Cl. GAMMA REACTIONS FOR DIRECT USPC ............................ 376/108: 376/112:376/156 ELECTRIC POWER GENERATION (58) Field of Classification Search USPC .......................... 376/157, 108,156, 171, 172 (76) Inventors: Charles S. Holden, San Francisco, CA See application file for complete search history. (US); Robert E. Schenter, Portland, OR (US) (56) References Cited *) Notice: Subject to anyy disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 3,748,226 A * 7/1973 Ribe et al. ..................... 376,124 U.S.C. 154(b) by 961 days. 3,778,627 A * 12/1973 Carpenter ...... ... 376, 192 4,749,540 A * 6/1988 Bogartet al. .. ... 376/133 (21) Appl. No.: 12/296,844 4,997,619 A * 3/1991 Pettus ............ ... 376,288 2004/02284.33 A1* 1 1/2004 Magill et al. ... 376/347 (22) PCT Filed: Apr. 13, 2007 2005/022O248 A1* 10, 2005 Ritter ...... ... 376/190 2008/O144762 A1* 6/2008 Holden et al. ..... ... 376/416 (86). PCT No.: PCT/US2OOTAO66668 * cited by examiner S371 (c)(1), (2), (4) Date: Oct. 10, 2008 Primary Examiner — Jack W Keith Assistant Examiner — Sean P Burke (87) PCT Pub. No.: WO2008/060663 (74) Attorney, Agent, or Firm — Craig M. Stainbrook; PCT Pub. Date: May 22, 2008 Stainbrook & Stainbrook, LLP (65) Prior Publication Data (57) ABSTRACT A neutron generator and isotope production apparatus and US 2011 FO268237 A1 Nov. -

The Discoverers of the Ruthenium Isotopes
•Platinum Metals Rev., 2011, 55, (4), 251–262• The Discoverers of the Ruthenium Isotopes Updated information on the discoveries of the six platinum group metals to 2010 http://dx.doi.org/10.1595/147106711X592448 http://www.platinummetalsreview.com/ By John W. Arblaster This review looks at the discovery and the discoverers Wombourne, West Midlands, UK of the thirty-eight known ruthenium isotopes with mass numbers from 87 to 124 found between 1931 and 2010. Email: [email protected] This is the sixth and fi nal review on the circumstances surrounding the discoveries of the isotopes of the six platinum group elements. The fi rst review on platinum isotopes was published in this Journal in October 2000 (1), the second on iridium isotopes in October 2003 (2), the third on osmium isotopes in October 2004 (3), the fourth on palladium isotopes in April 2006 (4) and the fi fth on rhodium isotopes in April 2011 (5). An update on the new isotopes of palladium, osmium, iridium and platinum discovered since the previous reviews in this series is also included. Naturally Occurring Ruthenium Of the thirty-eight known isotopes of ruthenium, seven occur naturally with the authorised isotopic abun- dances (6) shown in Table I. The isotopes were fi rst detected in 1931 by Aston (7, 8) using a mass spectrograph at the Cavendish Laboratory, Cambridge University, UK. Because of diffi cult experimental conditions due to the use of poor quality samples, Aston actually only detected six of the isotopes and obtained very approximate Table I The Naturally Occurring Isotopes of Ruthenium Mass number Isotopic Abundance, % 96Ru 5.54 98Ru 1.87 99Ru 12.76 100Ru 12.60 101Ru 17.06 102Ru 31.55 104Ru 18.62 251 © 2011 Johnson Matthey http://dx.doi.org/10.1595/147106711X592448 •Platinum Metals Rev., 2011, 55, (4)• percentage abundances. -

Metastable Non-Nucleonic States of Nuclear Matter: Phenomenology
Physical Science International Journal 15(2): 1-25, 2017; Article no.PSIJ.34889 ISSN: 2348-0130 Metastable Non-Nucleonic States of Nuclear Matter: Phenomenology Timashev Serge 1,2* 1Karpov Institute of Physical Chemistry, Moscow, Russia. 2National Research Nuclear University MEPhI, Moscow, Russia. Author’s contribution The sole author designed, analyzed and interpreted and prepared the manuscript. Article Information DOI: 10.9734/PSIJ/2017/34889 Editor(s): (1) Prof. Yang-Hui He, Professor of Mathematics, City University London, UK And Chang-Jiang Chair Professor in Physics and Qian-Ren Scholar, Nan Kai University, China & Tutor and Quondam-Socius in Mathematics, Merton College, University of Oxford, UK. (2) Roberto Oscar Aquilano, School of Exact Science, National University of Rosario (UNR),Rosario, Physics Institute (IFIR)(CONICET-UNR), Argentina. Reviewers: (1) Alejandro Gutiérrez-Rodríguez, Universidad Autónoma de Zacatecas, Mexico. (2) Arun Goyal, Delhi University, India. (3) Stanislav Fisenko, Moscow State Linguistic University, Russia. Complete Peer review History: http://www.sciencedomain.org/review-history/20031 Received 17 th June 2017 Accepted 8th July 2017 Original Research Article th Published 13 July 2017 ABSTRACT A hypothesis of the existence of metastable states for nuclear matter with a locally shaken-up nucleonic structure of the nucleus, was proposed earlier. Such states are initiated by inelastic scattering of electrons by nuclei along the path of weak nuclear interaction. The relaxation of such nuclei is also determined by weak interactions. The use of the hypothesis makes it possible to physically interpret a rather large group of experimental data on the initiation of low energy nuclear reactions (LENRs) and the acceleration of radioactive α- and β-decays in a low-temperature plasma. -

Oxidation-Reduction Alternating Copolymerization of Germylene and N-Phenyl-P-Quinoneimine
Polymer Journal (2015) 47, 31–36 & 2015 The Society of Polymer Science, Japan (SPSJ) All rights reserved 0032-3896/15 www.nature.com/pj ORIGINAL ARTICLE Oxidation-reduction alternating copolymerization of germylene and N-phenyl-p-quinoneimine Satoru Iwata1, Mitsunori Abe1, Shin-ichiro Shoda1 and Shiro Kobayashi1,2 The germylenes bis[bis(trimethylsilyl)amido]germanium (1a) and bis[t-butyl-trimethylsilyl]amido]germanium (1b) were reacted with N-phenyl-p-quinoneimine (2) to give copolymers (3a and 3b) with alternating tetravalent germanium and p-aminophenol units. The copolymerization took place smoothly at 0 °C without added catalyst or initiator. 1 acted as a reductant monomer, and 2 acted as an oxidant monomer (oxidation-reduction alternating copolymerization). Product copolymers were obtained in very high yields and had high molecular weights. The copolymers were soluble in toluene, benzene, n-hexane and chloroform, whereas they were insoluble in acetonitrile and acetone. Additionally, they were stable toward hydrolytic degradation. Electron spin resonance (ESR) spectroscopic studies of the reaction suggested a structure of a stable germyl radical and a plausible mechanism of biradical copolymerization. Polymer Journal (2015) 47, 31–36; doi:10.1038/pj.2014.84; published online 8 October 2014 INTRODUCTION investigations.10 Five- and six-membered cyclic germylenes, conver- Divalent germanium compounds (germylenes) and their tin analogs sely, were copolymerized with p-benzoquinone derivatives in similar (stannylenes) continue to attract -

First Observation of Alpha Decay of 190-Pt to the First Excited Level (E
First observation of α decay of 190Pt to the first excited 186 level (Eexc = 137.2 keV) of Os P. Bellia, R. Bernabeia,b,1, F. Cappellac,d, R. Cerullie, F.A. Danevichf , A. Incicchittic, M. Laubensteine, S.S. Nagornyf , S. Nisie, O.G. Polischukf , V.I. Tretyakf aINFN, Sezione di Roma “Tor Vergata”, I-00133 Rome, Italy bDipartimento di Fisica, Universita` di Roma “Tor Vergata” I-00133 Rome, Italy cINFN, Sezione di Roma “La Sapienza”, I-00185 Rome, Italy dDipartimento di Fisica, Universita` di Roma La “Sapienza”, I-00185 Rome, Italy eINFN, Laboratori Nazionali del Gran Sasso, 67010 Assergi (AQ), Italy f Institute for Nuclear Research, MSP 03680 Kyiv, Ukraine Abstract The alpha decays of naturally occurring platinum isotopes, which are accompanied by the emission of γ quanta, have been searched for deep underground (3600 m w.e.) in the Gran Sasso National Laboratories of the INFN (Italy). A sample of Pt with mass of 42.5 g and a natural isotopic composition has been measured with a low background HP Ge detector (468 cm3) during 1815 h. The alpha decay of 190Pt to the first excited 186 π + level of Os (J = 2 , Eexc = 137.2 keV) has been observed for the first time, with +0.4 ± × 14 the half-life determined as: T1/2 = 2.6−0.3(stat.) 0.6(syst.) 10 yr. The T1/2 limits for 16 20 the α decays of other Pt isotopes have been determined at level of T1/2 ≃ 10 − 10 yr. These limits have been set for the first time or they are better than those known from earlier experiments. -

5.696-Mev - I I 31-Mis 4 * 206 (Rihl Scale) - 400 ,— 400 - 5:94:5S\P
Lawrence Berkeley National Laboratory Recent Work Title ALPHA DECAY PROPERTIES OP NEUTRONS DEFICIENT ISOTOPES OF EMANATION Permalink https://escholarship.org/uc/item/2gr31474 Authors Nunnia, Matti J. Valli, Kalevi Hyde, Earl K. Publication Date 1966-05-01 eScholarship.org Powered by the California Digital Library University of California UCRL-16735 Rev. University of California Ernest 0. Lawrence Radiation Laboratory ALPHA DECAY PROPERTIES OF NEUTRON DEFICIENT ISOTOPES OF EMANATION TWO-WEEK LØAN COPY This is a library Circulating Copy which may be borróibed for two weeks. For a personal retention copy, call Tech. Info. DivIsIon, Ext. 5545 rk, California DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. II3 Supitte to Pc1 Revie; I9 UL16755 Rev. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide