The Course of Direct Projections of the Oculomotor Nucleus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optogenetic Fmri Interrogation of Brain-Wide Central Vestibular Pathways
Optogenetic fMRI interrogation of brain-wide central vestibular pathways Alex T. L. Leonga,b, Yong Guc, Ying-Shing Chand, Hairong Zhenge, Celia M. Donga,b, Russell W. Chana,b, Xunda Wanga,b, Yilong Liua,b, Li Hai Tanf, and Ed X. Wua,b,d,g,1 aLaboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Pokfulam, Hong Kong SAR, China; bDepartment of Electrical and Electronic Engineering, The University of Hong Kong, Pokfulam, Hong Kong SAR, China; cInstitute of Neuroscience, Key Laboratory of Primate Neurobiology, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China; dSchool of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China; eShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China; fCenter for Language and Brain, Shenzhen Institute of Neuroscience, Shenzhen 518057, China; and gState Key Laboratory of Pharmaceutical Biotechnology, The University of Hong Kong, Pokfulam, Hong Kong SAR, China Edited by Marcus E. Raichle, Washington University in St. Louis, St. Louis, MO, and approved March 20, 2019 (received for review July 20, 2018) Blood oxygen level-dependent functional MRI (fMRI) constitutes a multisensory integration process in the vestibular system is op- powerful neuroimaging technology to map brain-wide functions tokinetic nystagmus, whereby visual cues are used to induce in response to specific sensory or cognitive tasks. However, fMRI compensatory reflexive eye movements to maintain a stable gaze mapping of the vestibular system, which is pivotal for our sense of while moving (11, 12). These eye movements involve inputs from balance, poses significant challenges. -

Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience
CNs 5, 7, 9, 10 - Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience PHARYNGEAL ARCH SET, CNS 5, 7, 9, 10 • They are derived from the pharyngeal (aka branchial) arches • They have special motor and autonomic motor functions CRANIAL NERVES EXIT FROM THE BRAINSTEM CN 5, the trigeminal nerve exits the mid/lower pons.* CN 7, the facial nerve exits the pontomedullary junction.* CN 9, the glossopharyngeal nerve exits the lateral medulla.* CN 10, the vagus nerve exits the lateral medulla.* CRANIAL NERVE NUCLEI AT BRAINSTEM LEVELS Midbrain • The motor trigeminal nucleus of CN 5. Nerve Path: • The motor division of the trigeminal nerve passes laterally to enter cerebellopontine angle cistern. Pons • The facial nucleus of CN 7. • The superior salivatory nucleus of CN 7. Nerve Path: • CN 7 sweeps over the abducens nucleus as it exits the brainstem laterally in an internal genu, which generates a small bump in the floor of the fourth ventricle: the facial colliculus • Fibers emanate from the superior salivatory nucleus, as well. Medulla • The dorsal motor nucleus of the vagus, CN 10 • The inferior salivatory nucleus, CN 9 1 / 3 • The nucleus ambiguus, CNs 9 and 10. Nerve Paths: • CNs 9 and 10 exit the medulla laterally through the post-olivary sulcus to enter the cerebellomedullary cistern. THE TRIGEMINAL NERVE, CN 5  • The motor division of the trigeminal nerve innervates the muscles of mastication • It passes ventrolaterally through the cerebellopontine angle cistern and exits through foramen ovale as part of the mandibular division (CN 5[3]). Clinical Correlation - Trigeminal Neuropathy THE FACIAL NERVE, CN 7  • The facial nucleus innervates the muscles of facial expression • It spans from the lower pons to the pontomedullary junction. -

Direct Projections from Cochlear Nuclear Complex to Auditory Thalamus in the Rat
The Journal of Neuroscience, December 15, 2002, 22(24):10891–10897 Direct Projections from Cochlear Nuclear Complex to Auditory Thalamus in the Rat Manuel S. Malmierca,1 Miguel A. Mercha´n,1 Craig K. Henkel,2 and Douglas L. Oliver3 1Laboratory for the Neurobiology of Hearing, Institute for Neuroscience of Castilla y Leo´ n and Faculty of Medicine, University of Salamanca, 37007 Salamanca, Spain, 2Wake Forest University School of Medicine, Department of Neurobiology and Anatomy, Winston-Salem, North Carolina 27157-1010, and 3University of Connecticut Health Center, Department of Neuroscience, Farmington, Connecticut 06030-3401 It is known that the dorsal cochlear nucleus and medial genic- inferior colliculus and are widely distributed within the medial ulate body in the auditory system receive significant inputs from division of the medial geniculate, suggesting that the projection somatosensory and visual–motor sources, but the purpose of is not topographic. As a nonlemniscal auditory pathway that such inputs is not totally understood. Moreover, a direct con- parallels the conventional auditory lemniscal pathway, its func- nection of these structures has not been demonstrated, be- tions may be distinct from the perception of sound. Because cause it is generally accepted that the inferior colliculus is an this pathway links the parts of the auditory system with prom- obligatory relay for all ascending input. In the present study, we inent nonauditory, multimodal inputs, it may form a neural have used auditory neurophysiology, double labeling with an- network through which nonauditory sensory and visual–motor terograde tracers, and retrograde tracers to investigate the systems may modulate auditory information processing. -

Studies of Oculomotor-System Neural Connections Ronald S
Journal of the Arkansas Academy of Science Volume 34 Article 26 1980 How We Look: Studies of Oculomotor-System Neural Connections Ronald S. Remmel University of Arkansas for Medical Sciences Robert D. Skinner University of Arkansas for Medical Sciences Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Sense Organs Commons Recommended Citation Remmel, Ronald S. and Skinner, Robert D. (1980) "How We Look: Studies of Oculomotor-System Neural Connections," Journal of the Arkansas Academy of Science: Vol. 34 , Article 26. Available at: http://scholarworks.uark.edu/jaas/vol34/iss1/26 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 34 [1980], Art. 26 HOW WE LOOK: STUDIES OF OCULOMOTOR-SYSTEM NEURAL CONNECTIONS . R. S. REMMELandR. D. SKINNER i Department of Physiology and Biophysics and Department of Anatomy University of Arkansas forMedical Sciences Little Rock, Arkansas 72205 ABSTRACT The neural connections of the reticular formation (RF) withthe vestibular nuclei (8V)and the ascending medial longitudinal fasciculus (MLF) were studied, because many neurons in these structures carry eye-movement and head-movement (vestibular) signals and are only one or two synaptic connections removed from eye motorneurons. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Brainstem Dysfunction in Critically Ill Patients
Benghanem et al. Critical Care (2020) 24:5 https://doi.org/10.1186/s13054-019-2718-9 REVIEW Open Access Brainstem dysfunction in critically ill patients Sarah Benghanem1,2 , Aurélien Mazeraud3,4, Eric Azabou5, Vibol Chhor6, Cassia Righy Shinotsuka7,8, Jan Claassen9, Benjamin Rohaut1,9,10† and Tarek Sharshar3,4*† Abstract The brainstem conveys sensory and motor inputs between the spinal cord and the brain, and contains nuclei of the cranial nerves. It controls the sleep-wake cycle and vital functions via the ascending reticular activating system and the autonomic nuclei, respectively. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, impairment of consciousness, dysautonomia, and respiratory failure. The brainstem is prone to various primary and secondary insults, resulting in acute or chronic dysfunction. Of particular importance for characterizing brainstem dysfunction and identifying the underlying etiology are a detailed clinical examination, MRI, neurophysiologic tests such as brainstem auditory evoked potentials, and an analysis of the cerebrospinal fluid. Detection of brainstem dysfunction is challenging but of utmost importance in comatose and deeply sedated patients both to guide therapy and to support outcome prediction. In the present review, we summarize the neuroanatomy, clinical syndromes, and diagnostic techniques of critical illness-associated brainstem dysfunction for the critical care setting. Keywords: Brainstem dysfunction, Brain injured patients, Intensive care unit, Sedation, Brainstem -

Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens)
Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens) Lecture (13) ▪ Important ▪ Doctors Notes Please check our Editing File ▪ Notes/Extra explanation ه هذا العمل مب ين بشكل أسا يس عىل عمل دفعة 436 مع المراجعة { َوَم نْ يَ َت َو َ ّكْ عَ َلْ ا َّْلل فَهُ َوْ َحْ سْ ُ ُُْ} والتدقيق وإضافة المﻻحظات وﻻ يغ ين عن المصدر اﻷسا يس للمذاكرة ▪ Objectives At the end of the lecture, students should be able to: ✓ List the cranial nuclei related to occulomotor, trochlear, and abducent nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the site of emergence and course of these 3 nerves. ✓ Describe the important relations of oculomotor, trochlear, and abducent nerves in the orbit ✓ List the orbital muscles supplied by each of these 3 nerves. ✓ Describe the effect of lesion of each of these 3 nerves. ✓ Describe the optic nerve and visual pathway. Recall the how these nerves exit from the brain stem: Optic (does not exit from brain stem) Occulomotor: ventral midbrain (medial aspect of crus cerebri) Trochlear: dorsal midbrain (caudal to inferior colliculus) Abducent: ventral Pons (junction b/w pons & pyramid) Brain (Ventral view) Brain stem (Lateral view) Extra-Ocular Muscles 7 muscles: (ترفع جفن العين) .Levator palpebrae superioris 1- Origin: from the roof of the orbit (4) Recti muscles: *Rectus: ماشي على ( Superior rectus (upward and medially 2- الصراط (Inferior rectus (downward and medially 3- المستقيم 4- Medial rectus (medial) (medial) 5- Lateral rectus (lateral) How to remember the 2 فحركته muscles not supplied by نفس اسمه -اسمها عكس وظيفتها- :Oblique muscles (2) 6- Superior oblique (downward and laterally) Oblique: CN3? Superior oblique goes -1 منحرفOrigin: from the roof of the orbit 7- Inferior oblique (upward and laterally) up (superior) and turns around (oblique) a notch يمشي Origin: from the anterior floor or pulley and its supply is عكس كﻻمه NB. -

Brainstem Dysfunction in Critically Ill Patients
Benghanem et al. Critical Care (2020) 24:5 https://doi.org/10.1186/s13054-019-2718-9 REVIEW Open Access Brainstem dysfunction in critically ill patients Sarah Benghanem1,2 , Aurélien Mazeraud3,4, Eric Azabou5, Vibol Chhor6, Cassia Righy Shinotsuka7,8, Jan Claassen9, Benjamin Rohaut1,9,10† and Tarek Sharshar3,4*† Abstract The brainstem conveys sensory and motor inputs between the spinal cord and the brain, and contains nuclei of the cranial nerves. It controls the sleep-wake cycle and vital functions via the ascending reticular activating system and the autonomic nuclei, respectively. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, impairment of consciousness, dysautonomia, and respiratory failure. The brainstem is prone to various primary and secondary insults, resulting in acute or chronic dysfunction. Of particular importance for characterizing brainstem dysfunction and identifying the underlying etiology are a detailed clinical examination, MRI, neurophysiologic tests such as brainstem auditory evoked potentials, and an analysis of the cerebrospinal fluid. Detection of brainstem dysfunction is challenging but of utmost importance in comatose and deeply sedated patients both to guide therapy and to support outcome prediction. In the present review, we summarize the neuroanatomy, clinical syndromes, and diagnostic techniques of critical illness-associated brainstem dysfunction for the critical care setting. Keywords: Brainstem dysfunction, Brain injured patients, Intensive care unit, Sedation, Brainstem -

The Nucleus Prepositus Hypoglossi Contributes to Head Direction Cell Stability in Rats
The Journal of Neuroscience, February 11, 2015 • 35(6):2547–2558 • 2547 Systems/Circuits The Nucleus Prepositus Hypoglossi Contributes to Head Direction Cell Stability in Rats William N. Butler and XJeffrey S. Taube Dartmouth College, Hanover, New Hampshire 03755 Head direction (HD) cells in the rat limbic system fire according to the animal’s orientation independently of the animal’s environmental location or behavior. These HD cells receive strong inputs from the vestibular system, among other areas, as evidenced by disruption of their directional firing after lesions or inactivation of vestibular inputs. Two brainstem nuclei, the supragenual nucleus (SGN) and nucleus prepositus hypoglossi (NPH), are known to project to the HD network and are thought to be possible relays of vestibular information. Previous work has shown that lesioning the SGN leads to a loss of spatial tuning in downstream HD cells, but the NPH has historically been defined as an oculomotor nuclei and therefore its role in contributing to the HD signal is less clear. Here, we investigated this role by recording HD cells in the anterior thalamus after either neurotoxic or electrolytic lesions of the NPH. There was a total loss of direction-specific firing in anterodorsal thalamus cells in animals with complete NPH lesions. However, many cells were identified that fired in bursts unrelated to the animals’ directional heading and were similar to cells seen in previous studies that damaged vestibular- associated areas. Some animals with significant but incomplete lesions of the NPH had HD cells that were stable under normal conditions, but were unstable under conditions designed to minimize the use of external cues. -

The Accessory Optic System: Basic Organization with an Update on Connectivity, Neurochemistry, and Function
UC Irvine UC Irvine Previously Published Works Title The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function. Permalink https://escholarship.org/uc/item/3v25z604 Journal Progress in brain research, 151 ISSN 0079-6123 Authors Giolli, Roland A Blanks, Robert H I Lui, Fausta Publication Date 2005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Chapter 13 The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function Roland A. Giolli1, , , Robert H.I. Blanks1, 2 and Fausta Lui3 1Department of Anatomy and Neurobiology, University of California, College of Medicine, Irvine, CA 92697, USA 2Charles E. Schmidt College of Science, Florida Atlantic University, 777 Glades Rd., P.O. Box 3091, Boca Raton, FL 33431, USA 3Dipartimento di Scienze Biomediche, Sezione di Fisiologia, Universita di Modena e Reggio Emilia, Via Campi 287, 41100, Modena, Italy Available online 10 October 2005. Abstract The accessory optic system (AOS) is formed by a series of terminal nuclei receiving direct visual information from the retina via one or more accessory optic tracts. In addition to the retinal input, derived from ganglion cells that characteristically have large receptive fields, are direction-selective, and have a preference for slow moving stimuli, there are now well-characterized afferent connections with a key pretectal nucleus (nucleus of the optic tract) and the ventral lateral geniculate nucleus. The efferent connections of the AOS are robust, targeting brainstem and other structures in support of visual-oculomotor events such as optokinetic nystagmus and visual–vestibular interaction. This chapter reviews the newer experimental findings while including older data concerning the structural and functional organization of the AOS. -

Cranial Nerves
Cranial Nerves Cranial nerve evaluation is an important part of a neurologic exam. There are some differences in the assessment of cranial nerves with different species, but the general principles are the same. You should know the names and basic functions of the 12 pairs of cranial nerves. This PowerPage reviews the cranial nerves and basic brain anatomy which may be seen on the VTNE. The 12 Cranial Nerves: CN I – Olfactory Nerve • Mediates the sense of smell, observed when the pet sniffs around its environment CN II – Optic Nerve Carries visual signals from retina to occipital lobe of brain, observed as the pet tracks an object with its eyes. It also causes pupil constriction. The Menace response is the waving of the hand at the dog’s eye to see if it blinks (this nerve provides the vision; the blink is due to cranial nerve VII) CN III – Oculomotor Nerve • Provides motor to most of the extraocular muscles (dorsal, ventral, and medial rectus) and for pupil constriction o Observing pupillary constriction in PLR CN IV – Trochlear Nerve • Provides motor function to the dorsal oblique extraocular muscle and rolls globe medially © 2018 VetTechPrep.com • All rights reserved. 1 Cranial Nerves CN V – Trigeminal Nerve – Maxillary, Mandibular, and Ophthalmic Branches • Provides motor to muscles of mastication (chewing muscles) and sensory to eyelids, cornea, tongue, nasal mucosa and mouth. CN VI- Abducens Nerve • Provides motor function to the lateral rectus extraocular muscle and retractor bulbi • Examined by touching the globe and observing for retraction (also tests V for sensory) Responsible for physiologic nystagmus when turning head (also involves III, IV, and VIII) CN VII – Facial Nerve • Provides motor to muscles of facial expression (eyelids, ears, lips) and sensory to medial pinna (ear flap). -
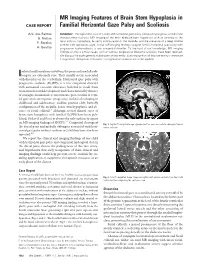
MR Imaging Features of Brain Stem Hypoplasia in Familial Horizontal
MR Imaging Features of Brain Stem Hypoplasia in CASE REPORT Familial Horizontal Gaze Palsy and Scoliosis A.V. dos Santos SUMMARY: We report the case of a child with horizontal gaze palsy, pendular nystagmus, and discrete S. Matias thoracolumbar scoliosis. MR imaging of the brain depicted pons hypoplasia with an absence of the facial colliculi, hypoplasia, butterfly configuration of the medulla, and the presence of a deep midline P. Saraiva pontine cleft (split pons sign). These MR imaging findings suggest familial horizontal gaze palsy with A. Goula˜o progressive kyphoscoliosis, a rare congenital disorder. To the best of our knowledge, MR imaging findings of only 4 similar cases, with or without progressive idiopathic scoliosis, have been reported. We discuss the pathogenesis substratum of this entity. Early recognition of this rare entity is important if supportive therapeutic measures in progressive scoliosis are to be applied. solated malformations involving the pons and medulla ob- Ilongata are extremely rare. They usually occur associated with disorders of the cerebellum. Horizontal gaze palsy with progressive scoliosis (HGPPS) is a rare congenital disorder with autosomal recessive inherence, believed to result from cranial nuclear maldevelopment and characterized by absence of conjugate horizontal eye movements, preservation of verti- cal gaze and convergence, progressive scoliosis developing in childhood and adolescence, midline pontine cleft, butterfly configuration of the medulla, brain stem hypoplasia, and ab- sence of facial colliculi.1 Although several clinical cases of brain stem hypoplasia with familial HGPPS have been pub- lished, Pieh et al and Rossi et al were the only authors to report on MR imaging findings of HGPPS.2,3 Congenital cleavage of Fig 1.