Antioxidant Capability Determination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Denominazione Ditta Alicave S.P.A. Aurecchia Giuseppe Buonanno Paolo Cannalonga Pietro Carlone Luigi Cave Alifane Ca.Ve. Dolomit
N_Pratic Denominazione_Ditta Materiale Comune 123 Alicave S.p.A. sabbia Alife 95 Aurecchia Giuseppe calcare Valle Agricola 270 Buonanno Paolo tufo Galluccio 27 Cannalonga Pietro calcare Pratella 434 Carlone Luigi calcare Dragoni 126 Cave Alifane calcare Alife 241 Ca.Ve. Dolomitica s.r.l. calcare Ailano 193 Cinotti Mario calcare San Potito Sannitico 319 Colacem (ex N.I.M.) calcare Ciorlano 478 D'Angelo Giuseppe pozzolana Marzano Appio 396 D'Angelo Pasquale pozzolana Teano 30 Delli Carpini Pasquale calcare Ciorlano 385 Di Gasparro Alberto calcare Presenzano 310 Edil Cave calcare S.Pietro Infine 274 Edil De Francesco pozzolana Teano 260 F.lli Leonardo calcare Roccaromana 342 Forang s.n.c. pozzolana Galluccio 349 Galdieri Wanda calcare Galluccio 247 Grassini Antonio Pomice Mignano Montelungo 575 Grassini Antonio Pomice Mignano Montelungo 29 Iacovone Francesco calcare Ciorlano 265 Iannotta Stelvio S.r.l. calcare Gioia Sannitica 358 Ispa sabbia Ruviano 394 Ispa sabbia Alife (Alvignano 25 Ital.cal calcare VairanoDra goni)Patenora 289 Italcementi S.p.A. tufo Marzano Appio 174 Izzo Pasquale e Mario pozzolana Sessa Aurunca 275 Landolfi Raffaele Inerti fluviali Gioia Sannitica 262 Leonardo Salvatore calcare Riardo 254 Moccia Irme S.p.A. Argilla Alvignano 144 Palomba Antonino calcare Alife 479 Pendolino Giuseppe calcare Liberi 26 Pro.mi.n. calcare Pietravairano 14 Promin (ex Fratelli Musto) Argilla Rocca D'Evandro 28 Roccio Giacinto calcare Ciorlano 347 Romanelli Ugo calcare Mignano Montelungo 122 S.P.L.di Caturano Pietro pozzolana Tora e Piccilli 363 San Giulianeta calcare Teano 33 Sangiuliano Michele calcare Prata Sannita 231 Tecnobeton Basalto Rocca D'Evandro 142 Testa Domenico calcare Alife 321 Venafrana Appalti calcare Ciorlano 322 Venafrana Inerti sabbia Capriati al Volturno 361 Viscovo Antonio calcare S.Pietro Infine 511 Visocchi Sconosciuto Ciorlano 364 Selcom s.r.l. -

Seggi Primarie 2015
COMUNE SEDE AILANO Circolo Pd -Piazza Dalla Chiesa ALIFE Aula Consiliare ALVIGNANO Aula Consiliare ARIENZO Circolo Pd - Via Unità d'Italia AVERSA 1 Circolo Pd - Piazza Municipio AVERSA 2 Ex Macello AVERSA 3 Ex Macello BAIA E LATINA Aula Consiliare - Piazza XX Settembre BELLONA Circolo Pd CAIANIELLO Atrio Palzza Municipale - Via S. Lucia CAIAZZO Circolo Pd - Via Melchiorri CALVI RISORTA Circolo Pd - Via Baroni CAMIGLIANO VOTA A PASTORANO CANCELLO ED ARNONE Sede Pd via Roma CAPODRISE Circolo Pd Piazza Massaro CAPRIATI A VOLTURNO Vota a prata CAPUA 1 Via Gran Priorato di Malta, 84 Circolo Pd fraz. San'Angelo in Formis - Via IV CAPUA 2 Novembre CARINARO Circolo Pd- Piazza Trieste CARINOLA Circolo Pd - fraz. Nocelleto Via IV Novembre CASAGIOVE Circolo Pd - Via Santa Croce CASAL DI PRINCIPE Circolo Pd - Via Santa Lucia CASALUCE Circolo Pd - Via G. Matteotti 74 CASAPESENNA Centro Sociale Cangiano CASAPULLA Circolo Pd CASERTA 1 Chiesa buon pastore Piazza Pitesti CASERTA 1 Chiesa buon pastore Piazza Pitesti CASERTA 2 Casa Comunale - piazza Vanvitelli CASERTA 3 Chiesa di Lourdes - Via Kennedy CASTELCAMPAGNANO Aula Consiliare CASTEL DI SASSO Vota a Formnicola CASTEL MORRONE Circolo Pd - Via taverna nuova, 45 CASTEL VOLTURNO 1 Circolo Pd - Via Fiume CASTEL VOLTURNO 2 Pinetamare piazzale Darsena CASTELLO MATESE Circolo Pd CELLOLE Aula consiliare CERVINO Vota a S: Maria A Vico CESA Circolo Pd - Via Marconi, 10 CIORLANO Vota ad Ailano CONCA DELLA CAMPANIA Vota a Mignano Montelungo CURTI Circolo Pd - piazza della Repubblica DRAGONI VOTA a Alvignano FALCIANO -

Commission Implementing Decision of 30 November 2018 on The
C 441/20 EN Official Journal of the European Union 7.12.2018 COMMISSION IMPLEMENTING DECISION of 30 November 2018 on the publication in the Official Journal of the European Union of the application for approval of an amendment, which is not minor, to a product specification referred to in Article 53 of Regulation (EU) No 1151/2012 of the European Parliament and of the Council for the name ‘Vitellone Bianco dell’Appennino Centrale’ (PGI) (2018/C 441/08) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EU) No 1151/2012 of the European Parliament and of the Council of 21 November 2012 on quality schemes for agricultural products and foodstuffs (1), and in particular Article 50(2)(a) in conjunction with Article 53(2) thereof, Whereas: (1) Italy has sent an application for approval of an amendment, which is not minor, to the product specification of ‘Vitellone Bianco dell’Appennino Centrale’ (PGI) in accordance with Article 49(4) of Regulation (EU) No 1151/2012. (2) In accordance with Article 50 of Regulation (EU) No 1151 /2012 the Commission has examined that application and concluded that it fulfils the conditions laid down in that Regulation. (3) In order to allow for the submission of notices of opposition in accordance with Article 51 of Regulation (EU) No 1151/2012, the application for approval of an amendment, which is not minor, to the product specification, as referred to in the first subparagraph of Article 10(1) of Commission Implementing Regulation (EU) No -

08 2 CV 2019 Ing. Pietro Terreri.Pdf
Curriculum Vitae Dott. Ing. Pietro Terreri INFORMAZIONI PERSONALI Dott. Ing. Pietro Terreri Residente in Formia (LT) alla via Ascatiello n.10, domiciliato in Piedimonte Matese (CE) alla via G. Matteotti n.54 08231767161 +39 3281186387 [email protected] ; [email protected] Sesso M | Data di nascita 20/01/1966 | Nazionalità Italiana POSIZIONE RICOPERTA Ingegnere, Libero Professionista, Dipendente Comunale a tempo indeterminato presso l’ Ente Comunale di Piedimonte Matese – Cat. D3 Iscritto all’Ordine degli Ingegneri della Provincia di Caserta al n.2311 dal 26/1/1999, proveniente dall’Ordine di Napoli, iscritto al n.11416, dal 3/4/1993 Iscritto all’albo del C.T.U. presso il Tribunale di Napoli col n.7748. E’ abilitato allo svolgimento dell’incarico di Coordinatore della Sicurezza ai sensi del Decreto Legislativo n. 494/96 poi D. Lgs 81/2008 dal Luglio 1999. TITOLO DI STUDIO Laurea in Ingegneria Civile sez. Trasporti presso L’università Degli Studi Federico II Di Napoli, con Luglio 1992. votazione 110/110. Tesi sperimentale in Costruzione di Ponti. ESPERIENZA PROFESSIONALE Maro 2008-attuale Dipendente a tempo indeterminato con Categoria D3 dell’Ente Comunale di Piedimonte Matese Ente Comunale Piedimonte Matese P.zza De Benedictis, 1 – Piedimonte Matese (CE) – www.comune.piedimontematese.ce.it ▪ Responsabile del Settore Territorio ed Ambiente e dell’Ufficio Sismico (marzo 2008 –settembre 2016) Nel corso di tale esperienza lavorativa ha svolto le funzioni direttive del settore con alle dipendenze, mediamente, n.6 addetti tra -

Publication of an Amendment Application Pursuant to Article 6(2
16.3.2011 EN Official Journal of the European Union C 82/7 OTHER ACTS EUROPEAN COMMISSION Publication of an amendment application pursuant to Article 6(2) of Council Regulation (EC) No 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs (2011/C 82/07) This publication confers the right to object to the amendment application pursuant to Article 7 of Council Regulation (EC) No 510/2006 ( 1). Statements of objection must reach the Commission within six months of the date of this publication. AMENDMENT APPLICATION COUNCIL REGULATION (EC) No 510/2006 AMENDMENT APPLICATION IN ACCORDANCE WITH ARTICLE 9 ‘VITELLONE BIANCO DELL'APPENNINO CENTRALE’ EC No: IT-PGI-0117-1552-26.10.2009 PGI ( X ) PDO ( ) 1. Heading in the product specification affected by the amendment: — Name of product — Description of product — Geographical area — Proof of origin — Method of production — Link — Labelling — National requirements — Other (to be specified) 2. Type of amendment(s): — Amendment to single document or summary sheet — Amendment to specification of registered PDO or PGI for which neither the single document nor the summary sheet has been published ( 1 ) OJ L 93, 31.3.2006, p. 12. C 82/8 EN Official Journal of the European Union 16.3.2011 — Amendment to specification that requires no amendment to the published single document (Article 9(3) of Regulation (EC) No 510/2006) — Temporary amendment to specification resulting from imposition of obligatory sanitary or phytosanitary measures by public authorities (Article 9(4) of Regulation (EC) No 510/2006) 3. Amendment(s): 3.1. -

Rankings Municipality of Valle Agricola
9/26/2021 Maps, analysis and statistics about the resident population Demographic balance, population and familiy trends, age classes and average age, civil status and foreigners Skip Navigation Links ITALIA / Campania / Province of Caserta / Valle Agricola Powered by Page 1 L'azienda Contatti Login Urbistat on Linkedin Adminstat logo DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA Municipalities Powered by Page 2 Ailano Stroll up beside >> L'azienda Contatti Login Urbistat on Linkedin Falciano del AdminstatAlife logo Massico DEMOGRAPHY ECONOMY RANKINGS SEARCH Alvignano ITALIA Fontegreca Arienzo Formicola Aversa Francolise Baia e Latina Frignano Bellona Gallo Matese Caianello Galluccio Caiazzo Giano Vetusto Calvi Risorta Gioia Sannitica Camigliano Grazzanise Cancello ed Gricignano di Arnone Aversa Capodrise Letino Capriati a Liberi Volturno Lusciano Capua Macerata Carinaro Campania Carinola Maddaloni Casagiove Marcianise Casal di Marzano Appio Principe Mignano Monte Casaluce Lungo Casapesenna Mondragone Casapulla Orta di Atella Caserta Parete Castel Pastorano Campagnano Piana di Monte Castel di Sasso Verna Castel Morrone Piedimonte Castel Volturno Matese Castello del Pietramelara Matese Pietravairano Cellole Pignataro Cervino Maggiore Cesa Pontelatone Ciorlano Portico di Powered by Page 3 Conca della Caserta L'azienda Contatti Login Urbistat on Linkedin Campania Prata Sannita Adminstat logo Curti DEMOGRAPHY ECONOMY RANKINGS SEARCH PratellaITALIA Dragoni Presenzano Raviscanina Recale Riardo Provinces Rocca d'Evandro Roccamonfina Roccaromana -

Aree Interne Caserta Litorale Domitio Aversa
0 1.5 3 6 9 12 15 Provincia di Caserta Piano territoriale di coordinamento provinciale Provincia di Caserta Km Ptc 400000 410000 420000 430000 440000 450000 460000 Rapporto ambientale ex art. 13 D.lgs 152/2006 CAPRIATI A VOLTURNO GALLO MATESE IS 0 0 0 0 Componente suolo 0 0 0 0 9 9 5 FONTEGRECA 5 4 4 Territorio aperto e territorio urbanizzato IS 9 SAN PIETRO INFINE LETINO CB data: gennaio 2010 CIORLANO PRATA SANNITA FR VALLE AGRICOLA SAN GREGORIO MATESE MIGNANO MONTE LUNGO PRATELLA PIEDIMONTE MATESE CASTELLO DEL MATESE AILANO ROCCA D'EVANDRO RAVISCANINA PRESENZANO 0 0 0 0 0 0 0 0 8 8 5 5 4 4 PIEDIMONTE MATESE CONCA DELLA CAMPANIA SANT'ANGELO D'ALIFE SAN POTITO SANNITICO GALLUCCIO TORA E PICCILLI VAIRANO PATENORA Aree interne PIETRAVAIRANO ALIFE MARZANO APPIO Legenda GIOIA SANNITICA CAIANELLO BAIA E LATINA Confine provinciale ROCCAMONFINA ! ! ! ! ! ! ! ! Confine comunale 0 0 0 0 0 LT 0 0 0 7 7 Confine ambito 5 5 4 4 ROCCAROMANA RIARDO PIETRAMELARA DRAGONI Uso del suolo Territori boscati e ambienti seminaturali TEANO ALVIGNANO SESSA AURUNCA Territori agricoli ROCCHETTA E CROCE LIBERI Territori modellati artificialmente: tessuto urbano storico FORMICOLA BN Territori modellati artificialmente: tessuto urbano recente RUVIANO GIANO VETUSTO CELLOLE CALVI RISORTA Uso del suolo Stato di fatto Ambito insediativo Uso del suolo per macro-categorie [ha] 0 0 territ. boscati territori territori Totale 0 0 0 0 0 CARINOLA 0 e ambienti agricoli modellati 6 6 5 CASTEL DI SASSO 5 4 4 PONTELATONE seminaturali artificialmente CAMIGLIANO CAIAZZO SPARANISE Caserta 15,650 41,550 11,000 68,200 Aversa 180 14,920 4,750 19,850 PIGNATARO MAGGIORE PIANA DI MONTE VERNA Litorale D. -

Ai Dirigenti Scolastici Delle Scuole Medie E Istituti Comprensivi Della Provincia LORO SEDI All’ALBO E Sito WEB SEDE Alle OO.SS
MIUR.AOOUSPCE.REGISTRO UFFICIALE(U).0017000.13-10-2016 Ministero dell’Istruzione, dell’Università e della Ricerca Ufficio IX – Ambito Territoriale di Caserta email: [email protected] - pec: [email protected] Tel. 0823.216413 – C.F. 80100690611 Ai Dirigenti Scolastici delle Scuole Medie e Istituti Comprensivi della provincia LORO SEDI All’ALBO e Sito WEB SEDE Alle OO.SS. LORO SEDI Oggetto: Disponibilità I Grado dopo l’effettuazione delle operazioni di mobilità annuale. Classi di concorso. Si dispone la pubblicazione delle disponibilità per classi di concorso nella Scuola Media per l’anno scolastico 2016/2017 dopo l’effettuazione delle operazioni di mobilità annuale. I Dirigenti Scolastici in indirizzo, oltre a curarne la massima diffusione, vorranno verificare la correttezza dei dati pubblicati e far presente eventuali difformità, con cortese urgenza, alla Sezione I Grado di questo Ufficio all’indirizzo peo [email protected]. IL DIRIGENTE F.to Vincenzo ROMANO Documento firmato digitalmente ai sensi del c.d. Codice dell’Amministrazione Digitale e normativa connessa Domenico MARINO Organici e Mobilità Personale Docente 1° Grado Tel: 0823248216 Mail: [email protected] DISPONIBILITA' STRUMENTO MUSICALE DOPO I MOVIMENTI ANNUALI 2016/2017 - SCUOLA MEDIA Codice Denominazione Comune CHITARRA SANTA MARIA CAPUA CEMM83701P R.UCCELLA -S.MARIA C.V.- VETERE 1 catt aspett. CEMM85901G GIOVANNI XXIII -RECALE- RECALE 1 catt ut licei 1 catt ut licei AP //////////////////////////////////////////////////////////////////////////// CEMM86701E G.MAZZINI -S.NICOLA LA STRADA- SAN NICOLA LA STRADA Pullone CEMM88601X "DE FILIPPO" S.NICOLA LA STRADA SAN NICOLA LA STRADA 1 catt ut licei CEMM89401V - V. ROCCO - SANT'ARPINO SANT'ARPINO 1 catt org CEMM89601E SCUOLA "PASCOLI" -CASAPESENNA- CASAPESENNA 1 catt ut licei CEMM8A4012 L. -

Il Percorso Il Massiccio Del Matese È Uno Dei Più Importanti Gruppi
Club Alpino Italiano ________________________________________________________________________________ SEZIONE DI NAPOLI DATA Domenica 11 Novembre 2018 ESCURSIONE INTERSEZIONALE COL CAI DI PIEDIMONTE MATESE Da Valle Agricola a Letino – lungo l’antica via di collegamento fra i due borghi Parco Nazionale del Matese – settore occidentale Quota massima: 1076 metri Dislivello totale (considerando i saliscendi): circa 550 metri Durata: ore 6 circa Difficoltà: E Equipaggiamento: scarponi, giacca a vento antipioggia, bastoncini, abbigliamento a strati da montagna. Colazione ed acqua: da portare Mezzi di trasporto: auto proprie (oppure autobus al raggiungimento del numero utile) Accompagnatori: Giuliana Alessio (339 6545655), Antonio Fiorentino (333 7373268), Pio Ciliberti (338 5622916), Gerardo Bertozzi (368 404816) Il Percorso Il massiccio del Matese è uno dei più importanti gruppi montuosi dell’Appennino meridionale, situato a cavallo tra Molise e Campania. E’ un territorio ricco di luoghi selvaggi, paesaggi dolci con laghi, ricco di splendide foreste, centri storici originali e ottimamente conservati. La nostra escursione si svolge in ambiente montano lungo l’antica via di collegamento tra i borghi di Valle Agricola e Letino nei Monti del Matese. Letino (Ru Tinu in dialetto letinese) è un comune italiano di 707 abitanti della provincia di Caserta in Campania Nel primo tratto si attraversa il paese di Valle Agricola, quindi si inizia una salita graduale lungo una sterrata in costante direzione sud-est verso nord-ovest lungo il versante meridionale dei Monti Capello e Cappello. Tale sterrata in seguito diventa un suggestivo sentiero con direzione da sud- ovest a nord-est, immerso in ambiente roccioso, caratterizzato da bellissimi panorami verso ovest. Raggiunto il valico a quota 1076 m, inizia una discesa lungo il bosco per raggiungere prima lo spettacolare affaccio sulla sottostante Rava di Prata e poi si continua la discesa fino al lago di Letino, dove si potrà effettuare la sosta pranzo. -

Comune Di Valle Agricola Provincia Di Caserta Piano D'emergenza
Piano d’Emergenza Comunale di Valle Agricola___________ ________________ Rischio Incendio Boschivo COMUNE DI VALLE AGRICOLA PROVINCIA DI CASERTA PIANO D’EMERGENZA COMUNALE RISCHIO INCENDIO BOSCHIVO Aggiornamento Piano di Emergenza, Comunale alle vigenti indicazioni operative adottate dal Dipartimento della Protezione Civile e delle linee guida approvate dalla Giunta Regionale della Campania con propria deliberazione n.146 del 27/05/2013. 1 Piano d’Emergenza Comunale di Valle Agricola___________ ________________ Rischio Incendio Boschivo INDICE RISCHIO INCENDIO BOSCHIVO …………………………………………………………………………………………...……. p. 3 La popolazione e il territorio ……………………………………………………………………………………………………...…. p. 3 Dati climatici ……………………………………………………………………………………………………………………….... p. 3 Dati incendi boschivi ……………………………………………………………………………………………………………….... p. 3 LINEAMENTI DELLA PIANIFICAZIONE …………………………………………………….…………………………………. p. 5 Lineamenti della Pianificazione nei Periodi di non Emergenza …………………………………………..…………………………. p. 5 Lineamenti della pianificazione nei periodi di emergenza ………………………………………..…………………………………. p. 6 MODELLI DI INTERVENTO ……………………………………………………………………………………………………… p. 9 MODELLI DI INTERVENTO NEI PERIODI DI NON EMERGENZA ………………………………………..……..…...……. p. 10 ALLEGATO A’IB - INFORMAZIONE ALLA POPOLAZIONE ………………………………………………………………... p. 10 ALLEGATO B’IB - ESERCITAZIONI PERIODICHE ……………………………………….……………………………..……. p. 10 ALLEGATO C’IB - MANUTENZIONE E CONTROLLO DELLE AREE STRATEGICHE ………………………………..…… p. 11 ALLEGATO D’IB - MANUTENZIONE E CONTROLLO DELLA VIABILITÀ -
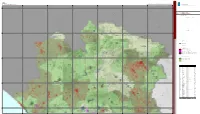
B5.2.1 Le Tipologie Insediative
0 1 2 4 6 8 10 Provincia di Caserta Km Provincia di Caserta Ptc 400000 410000 420000 430000 440000 450000 460000 Piano territoriale di coordinamento provinciale 0 0 0 0 0 0 0 0 0 0 6 6 4 4 Territorio insediato IS B5.2.1 Le tipologie insediative data: settembre 2009 Inquadramento tavole__quadrante Nord CB CAPRIATI A VOLTURNO GALLO MATESE scala: 1:50.000 0 0 0 0 0 0 0 0 9 FR 9 5 5 4 FONTEGRECA 4 SAN PIETRO INFINE LETINO CIORLANO Legenda PRATA SANNITA Confine provinciale VALLE AGRICOLA ! ! ! ! ! ! Confine comunale SAN GREGORIO MATESE MIGNANO MONTE LUNGO Tipologie insediative PRATELLA Tessuto urbano storico (3.354 ha) Tessuto urbano non residenziale (6.757 ha) PIEDIMONTE MATESE Tessuto urbano recente realizzato in presenza di Prg (3.094 ha) CASTELLO DEL MATESE Tessuto urbano recente realizzato in assenza di Prg (12.246 ha) AILANO ROCCA D'EVANDRO RAVISCANINA PRESENZANO Pianificazione urbanistica 0 0 0 0 0 0 0 0 8 8 5 5 4 4 Comuni provvisti di Prg entro il 1979 PIEDIMONTE MATESE Comuni provvisti di Prg entro il 1999 Comuni sprovvisti di Prg al 1999 SANT'ANGELO D'ALIFE CONCA DELLA CAMPANIA SAN POTITO SANNITICO GALLUCCIO TORA E PICCILLI Stato della pianificazione urbanistica comunale Decreto di Cod.Istat Comune Stato della pianificazione urbanistica Data di VAIRANO PATENORA approvazione approvazione 61001 Ailano Prg approvato D.P.G.R.C 227/2003 61002 Alife(*) Prg approvato D.P.G.R.C. 2490/1976 61003 Alvignano Pdf approvato D.P.G.R.C. 2026/1976 61006 Baia e Latina Prg approvato (variante) D.D.R.C. -

Gabinetto1.Pdf
ARCHIVIO DI STATO DI CASERTA Prefettura di Caserta GABINETTO Numero Numero busta fascicolo Oggetto Data 1 1 Riduzione di palchi del teatro di Caserta a disposizione delle autorità 1862 dic.6 - 1863 dic.5 1 2 Suppliche di carcerati per ottenere la libertà 1863 ott.4 - 1863 dic.4 1 3 Tumulti avvenuti nella casa di reclusione maschile di Aversa 1867 ago.2 - 1867 set.7 Numero Numero busta fascicolo Oggetto Data 1 4 Notizie sui reclusi negli stabilimenti penali della provincia 1869 mar.1 - 1869 Ap.12 Relazione sullo stato morale e materiale degli stabilimenti di pena di Aversa, Gaeta, Ponza 1870 gen.15 - 1871 ott.18 1 5 Matricola dei servizi e informazioni del personale amministrativo, sanitario e religioso delle 1870 ott.5 - 1872 feb.20 1 6 carceri e del sifilicomio 1 7 Irregolarità nel servizio disciplinare nella casa di pena di Aversa 1871 giu.20 - 1871 nov.4 Relazione annuale sul servizio nelle case di pena di Aversa e Gaeta, S. Maria C.V. e Ponza 1872 gen.31 - 1872 apr.9 1 8 1 9 Reclamo dei detenuti della casa di forza di Aversa 1872 giu.17 - 1872 ago.13 1 10 Ammutinamento nella prigione di S. Maria C.V. 1873 giu.3 - 1873 giu.21 Circolare ministeriale circa gli onori da rendersi ai prefetti da parte delle autorità militari 1873 giu.13 - 1 11 1 12 Notizie sul personale amministrativo, sanitario e religioso delle carceri e sifilicomi 1873 lug.9 - 1873 nov.8 1 13 Inchiesta per abusi negli appalti di lavori nelle carceri di Ponza 1874 dic.31 - 1875 gen.11 1 14 Circolare per l'attuazione del segreto di ufficio 1875 nov.8 - 1876 feb.8 2 15