Chromosomal Aberrations, Sister Chromatid Exchanges and Nuclear Status of Immature Oocytes in Relation to Age of Dromedary Camels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

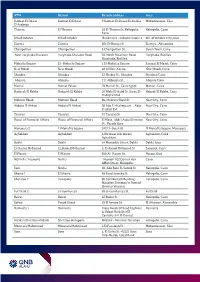
Importers Address Telephone Fax Make(S)
Importers Address Telephone Fax Make(s) Alpha Auto trading Josef tito st. Cairo +20 02-2940330 +20 02-2940600 Citroën cars Amal Foreign Trade Heliopolis, Cairo 11Fakhry Pasha St +20 02-2581847 +20 02-2580573 Lada Artoc Auto - Skoda 2, Aisha Al Taimouria st. Garden city Cairo +20 02-7944172 +20 02-7951622 Skoda Asia Motors Egypt 69, El Nasr Road, New Maadi, Cairo +20 02-5168223 +20 02-5168225 Asia Motors Atic/Arab Trading & 21 Talaat Harb St. Cairo +20 02-3907897 +20 02-3907897 Renault CV Insurance Center of 4, Wadi Al nil st. Mohandessin Cairo +20 02-3034775 +20 02-3468300 Peugeot Development & commerce - CDC - Wagih Abaza Chrysler Egypt 154 Orouba St. Heliopolis Cairo +20 02-4151872 +20 02-4151841 Chrysler Daewoo Corp Dokki, Giza- 18 El-Sawra St. Cairo +20 02-3370015 +20 02-3486381 Daewoo Daimler Chrysler Sofitel Tower, 28 th floor Conish el Nil, +20 02-5263800 +20 02-5263600 Mercedes, Egypt Maadi, Cairo Chrysler Egypt Engineering Shubra, Cairo-11 Terral el-ismailia +20 02-4266484 +20 02-4266485 Piaggio Industries Egyptan Automotive 15, Mourad St. Giza +20 02-5728774 +20 02-5733134 VW, Audi Egyptian Int'l Heliopolice Cairo Ismailia Desert Rd: Airport +20 02-2986582 +20 02-2986593 Jaguar Trading & Tourism / Rolls Royce Jaguar Egypt Ferrari El-Alamia ( Hashim Km 22 First of Cairo - Ismailia road +20 02-2817000 +20 02-5168225 Brouda Kancil bus ) Engineering Daher, Cairo 11 Orman +20 02-5890414 +20 02-5890412 Seat Automotive / SMG Porsche Engineering 89, Tereat Al Zomor Ard Al Lewa +20 02-3255363 +20 02-3255377 Musso, Seat , Automotive Co / Mohandessin Giza Porsche SMG Engineering for Cairo 21/24 Emad El-Din St. -

Directory of Development Organizations
EDITION 2010 VOLUME I.A / AFRICA DIRECTORY OF DEVELOPMENT ORGANIZATIONS GUIDE TO INTERNATIONAL ORGANIZATIONS, GOVERNMENTS, PRIVATE SECTOR DEVELOPMENT AGENCIES, CIVIL SOCIETY, UNIVERSITIES, GRANTMAKERS, BANKS, MICROFINANCE INSTITUTIONS AND DEVELOPMENT CONSULTING FIRMS Resource Guide to Development Organizations and the Internet Introduction Welcome to the directory of development organizations 2010, Volume I: Africa The directory of development organizations, listing 63.350 development organizations, has been prepared to facilitate international cooperation and knowledge sharing in development work, both among civil society organizations, research institutions, governments and the private sector. The directory aims to promote interaction and active partnerships among key development organisations in civil society, including NGOs, trade unions, faith-based organizations, indigenous peoples movements, foundations and research centres. In creating opportunities for dialogue with governments and private sector, civil society organizations are helping to amplify the voices of the poorest people in the decisions that affect their lives, improve development effectiveness and sustainability and hold governments and policymakers publicly accountable. In particular, the directory is intended to provide a comprehensive source of reference for development practitioners, researchers, donor employees, and policymakers who are committed to good governance, sustainable development and poverty reduction, through: the financial sector and microfinance, -

"Clouds in Egypt's Sky"
"Clouds in Egypt's Sky" Sexual Harassment: from Verbal Harassment to Rape A Sociological Study Scientific revision by Prepared by Dr. Aliyaa Shoukry Rasha Mohammad Hassan Professor of Anthropological Sociology ECWR Researcher Supervisor Nehad Abul Komsan ECWR Chair 1 CONCLUSIONS The issue of sexual harassment has become less taboo recently in the Egyptian media and within academic circles, and has even become a part of daily discourse among women in Egyptian society, regardless of social or economic status or political belief. In the past, women were afraid to talk about sexual harassment and considered discussing it culturally taboo. With the problem worsening, we have found that the way ahead is to encourage dialogue about this problem and to try to search for solutions. Sexual harassment has become an overwhelming and very real problem experienced by all women in Egyptian society, often on a daily basis, in public places such as markets, public transportation and the streets, as well as in private places such as educational institutions, sports clubs, and the workplace. The research component of the Egyptian Center for Women’s Rights’ (ECWR) work concerning the phenomenon of sexual harassment has progressed in three phases. In 2005 we received and documented over 100 complaints of women subjected to sexual harassment – women across all different age groups and socio-economic classes. These women spoke to us about the seriousness of the problem and the extent of their personal and private suffering. During the second phase of our research, in order to investigate the issue further and discover if the problem of sexual harassment was an isolated phenomenon or a pervasive problem faced by the majority of women in Egypt, we conducted an exploratory study surveying over 2,800 Egyptian women. -

Information for Asylum-Seekers and Refugees in Egypt
UNHCR The UN Refugee Agency Information For Asylum-Seekers and Refugees in Egypt United Nations High Commissioner for Refugees Regional Representation in Egypt Cairo, April 2013 CONTENTS Page INTRODUCTION 4 PART ONE: UNHCR MANDATE AND ITS ROLE IN THE ARAB REPUBLIC OF EGYPT 5 1.1 UNHCR Mandate 5 1.2 UNHCR Role in the Arab Republic of Egypt 7 PART TWO: RECEPTION AND GENERAL OFFICE PROCEDURES 11 2.1 Reception 11 2.2 General Office Procedures 13 2.3 Code of Conduct 18 PART THREE: REGISTRATION AND DOCUMENTATION FOR REFUGEES AND ASYLUM SEEKERS 22 3.1 Registration Process 22 3.2 Documentation-Process 29 PART FOUR: REFUGEE STATUS DETERMINATION PROCESS 42 4.1 Refugee Status Determination (RSD interview) 42 4.2 Legal Aid / Representation 45 4.3 Notification of RSD decisions 46 2 4.4 Appeal process 50 4.5 Cancellation and cessation of refugee status 54 4.6 Re-opening requests 56 4.7 Family unity 58 PART FIVE: LEGAL PROTECTION 64 PART SIX: ACCESS TO ASYLUM RIGHTS 66 6.1 Access to Health Care 66 6.2 Access to Education 73 6.3 Psycho-Social support at community level 79 6.4 Access to community based services 81 PART SEVEN: MEANS OF LIVELIHOOD 83 7.1 Means of live lihood 83 7.2 Vocational training 85 PART EIGHT: FINANCIAL ASSISTANCE 87 PART NINE: DURABLE SOLUTIONS 90 9.1 Voluntary Repatriation 90 9.1.1 Return to South Sudan 94 9.1.2 Return to the Sudan 97 9.1.3 Return to Iraq 98 9.2 Local Integration 101 9.3 Resettlement 102 PART TEN: UNHCR CAIRO COMPLAINTS PROCEDURES 109 PART ELEVEN: USEFUL CONTACTS 113 3 INTRODUCTION The purpose of this information booklet is to provide an overview of the mandate of the United Nations High Commissioner for Refugees (UNHCR) and the relevant criteria and procedures that are implemented by UNHCR in Egypt. -

List of Medical Doctors
Embassy of Switzerland in Egypt List of Medical Doctors/Therapists / ÄRZTELISTE NACH FACHGEBIET / Liste des médecins par spécialité Last update: 02/2020 (This list is being released with neither the endorsement nor the guarantee of the Embassy) Embassy’s medical doctor / Vertrauensarzt / Médecin de confiance Embassy’s medical doctor: Tel : +202 3338 2393 Address: Tel : +202 3761 1797 2, El-Fawakeh Street, Mohandessin, Dr. Abdel Meguid KASSEM near Moustafa Mahmoud Mosque -Giza. specialty: Gastroenterology, Hepatology, Infections Mobile : +20 100 176 8255 Hours: German, English, Arabic [email protected] Sunday - Wednesday [email protected] 17h30 – 20h00 Dr. Cherine KAHIL Tel: + 202 27 35 83 84 Home visit French, English , Arabic Mobile : +20 122 218 2279 [email protected] Dr. Sabine KLINKE Work location FDFA, Company Medical Officer Tel. : +41 58 481 4536 Freiburgstrasse 130, Bern, CH [email protected] Office no. A-2247 Medical Doctor (Internist) / ALLGEMEINMEDIZINER / Médecine générale (interniste) Dr. Sherif Doss Tel: +202 2358 3105 Address: English, Arabic Clinic Dr. Sherif DOSS Mobile : +20 122 210 3473 87, road 9 (Floor No. 5 ) Maadi [email protected] Clinic Hours Sunday, Tuesday, Wednesday 13h00 - 15h00 Dr. Ramez Guindy Cairo Specialized Hospital Address: English, Arabic Heliopolis, 4 Abou Ebaid El Bakry St. Internist Doctor & Cardiology Mobile : +20 122 215 8305 Off Ghernata St; Roxy- Heliopolis Tel : +202 2450 9800 ext 234 Hours [email protected] Saturday – Monday – Wednesday : 11h00 – 14h00 Sunday – Tuesday – Thursday : 15h00 – 17h00 Italian Hospital & Ain Shams Address: Reservation number : 17, El Sarayat St., El Abbasia - Cairo - Egypt +20 122 022 6501 Hours Sunday – Tuesday – Thursday : 10h00 - 12h30 Dr. -

Egypt’S Public Prosecutor Ordered the Supreme National Security Prosecution to Launch Investigations on the Incident of the Raised Flags During the Concert
HAUT-COMMISSARIAT AUX DROITS DE L’HOMME • OFFICE OF THE HIGH COMMISSIONER FOR HUMAN RIGHTS PALAIS DES NATIONS • 1211 GENEVA 10, SWITZERLAND Mandates of the Working Group on Arbitrary Detention; the Special Rapporteur on the promotion and protection of the right to freedom of opinion and expression; the Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health; the Special Rapporteur on the situation of human rights defenders; the Special Rapporteur on the right to privacy; and the Independent Expert on protection against violence and discrimination based on sexual orientation and gender identity REFERENCE: UA EGY 17/2017 31 October 2017 Excellency, We have the honour to address you in our capacity as Working Group on Arbitrary Detention; Special Rapporteur on the promotion and protection of the right to freedom of opinion and expression; Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health; Special Rapporteur on the situation of human rights defenders; Special Rapporteur on the right to privacy; and Independent Expert on protection against violence and discrimination based on sexual orientation and gender identity, pursuant to Human Rights Council resolutions 33/30, 34/18, 33/9, 34/5, 28/16 and 32/2. In this connection, we would like to bring to the attention of your Excellency’s Government information we have received concerning the alleged unlawful arrests and detention, as well as incrimination of persons based on their actual or perceived sexual orientation or gender identity and expression, and/or their actual or perceived expression and advocacy for protection of the human rights of LGBT people, including of two human rights defenders, Mr. -
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St. -

Informal Ties, Social Capital and Development: Popular Committees in Egypt, a Case Study Post-‐25Th of January Revolution”
The American University in Cairo School of Humanities and Social Sciences “Informal ties, Social Capital and development: Popular Committees in Egypt, a Case Study Post-‐25th of January Revolution” A Thesis Submitted to The Department of Political Science in partial fulfilment of the requirements for the degree of Master of Arts by Lamyaa Khaled Rayan Feb. /2013 Error! Unknown switch argument. The American University in Cairo “Informal ties, Social Capital and development: Popular Committees in Egypt, a Case Study Post-‐25th of January Revolution” A Thesis Submitted by Lamyaa Khaled Rayan To Department of (Political Sciences) Feb. /2013 In partial fulfilment of the requirements for The degree of Master of Arts Has been approved by Dr. Mustapha Kamel Al-Sayyid Thesis Committee Advisor --------------------------------------------- Dr. Clement Henry Thesis Committee Reader ----------------------------------------------- Dr. Nadine Sika Thesis Committee Reader------------------------------------------------- _________________ _______ ______________ _______ Department Chair Date Dean Date ACKNOWLEDGMENTS First and foremost, I offer my utmost gratitude to my advisor Dr. Mustapha Kamel Al-Sayyid for his intellectual guidance and keen encouragement to conduct my academic research and to complete the thesis. I would like to also thank the committee readers, Dr. Clement Henry and Dr. Nadine Sika for their critical insights, and their constructive suggestions to improve this work. I also greatly thank the Political science department’s Student Liaison Officer, Mrs. Dina Hosni for accommodating many of my inquires throughout my study years, giving valuable advice, and facilitating administrative difficulties. I would like to thank my family for their patience and continuous support. With their belief in me, I am forever indebted to them. -

List of Pharmacies
Pharmacies in Egypt (Updated July 2020) The Embassy of the United States of America in Cairo assumes no responsibility for the professional ability or integrity of the persons or firms whose names appear in the following list. The names listed are arranged alphabetically, and the order in which they appear has no other significance. Note: The number between brackets is the city code for landline phone numbers. For example, (02) is for Cairo, (03) for Alexandria, and so on. When calling any landline number within Egypt from a mobile phone you must include the city code. When calling from a landline within Egypt you must include the city code if you are calling a landline in any city except the one where the landline you are using is located. CAIRO DOKKI Ezz Eldeen Hotline: 15055 252 Sudan St., Lebanon Square, Dokki, Giza Open 24 hours a day We Care Mobile: 0111-967-6661 128 Ali Abd El-Haleem, Boulaq Num.2, Dokki, Giza Open 24 hours a day 19011 Hotline: 19011 31 Wezaret Al Zeraa St., Beside CBM, Dokki, Giza Open 24 hours a day DOWNTOWN/ Ali & Ali Pharmacy Tel: (02) 2368-2169 GARDEN CITY 33 Kasr El Aini Street (02) 2365-3880 Open 24 hours a day El Ezaby Tel: (02) 2795-2311 58 Kasr El Aini Street (02) 2795-6244 Open 24 hours a day Hakim Pharmacy Tel: (02) 2794-0403/ 4 Latin America St., in front of US Embassy, Garden 4204 City Open 9am- 08pm FIFTH El Ezaby Hotline: 19600 SETTLEMENT Shop No. R1.07, Downtown Mall, St Ninety, Fifth Open 24 hours a day Settlement Ezz Eldin Hotline: 15055 Shop No. -

Appendix 3.2 Description of Survey Tasks
APPENDIX 3.2 DESCRIPTION OF SURVEY TASKS APPENDIX 3.2 DESCRIPTION OF SURVEY TASKS 1. Preparatory Work The candidate survey locations were visited several times to: • Select a suitable location for each survey station. • Determine the required manpower of surveyors and supervisors • Sketch the site and its surrounding features. The following precautions were considered when selecting the survey locations: • The survey site has to be on a straight part of the road to provide sufficient sight distance for the surveyor to see the coming vehicles and to secure the safety of the survey team. • The survey site should be on a level road section to avoid the increase in vehicle speed in the down-grade direction which may increase the hazards possibility against the survey team. • The survey site has to be on an illuminated section as possible to provide adequate vision to the survey team during dark periods of the survey works and maintain safety aspects to the survey team. • The survey site has to be easily accessible by optimizing the transport process of the survey team to/from each site. • A detailed sketch for each survey station site should be prepared by the site supervisors. (1) Locations of Traffic Count Survey A total of 17 traffic count stations were, originally, selected to carry out the manual classified count (MCC) for two days during 18 hours starting from 6:00 A.M. till 12:00 A.M. These count stations can be classified into two major categories. The first category (10 bridges) is represented by a screenline along the Nile River. -

144-156, 2012 ISSN 1819-544X This Is a Refereed Journal and All Articles Are Professionally Screened and Reviewed
144 Journal of Applied Sciences Research, 8(1): 144-156, 2012 ISSN 1819-544X This is a refereed journal and all articles are professionally screened and reviewed ORIGINAL ARTICLES An Approach towards Solving Pedestrian Problems in Modern Cairo Nihal Mohamed Maarouf and Ayman Hisham El-Alfy Department of Civil & Architectural Engineering. National Research Center, Dokki, Giza, Egypt. ABSTRACT This study concerns pedestrians' needs fulfillment in one of the most important Boulevards in Greater Cairo, the capital and the largest city in Egypt and the eleventh biggest city in the world. This site represents heavy vehicle and pedestrian movement along one of the biggest sidewalks in Cairo, called Boulevard of Gameat Al Dowal. The study contains information -using a descriptive analytical approach- on physical characteristics of the shopping area served by the pedestrian path along the well known boulevard. Pedestrians' patterns of using the street and needs are investigated. At the end, relying on pedestrians sidewalk problems identification, a solution was introduced in order to regenerate, revitalize and improve walking conditions in this prominent public urban space. The most significant problems found were: the unevenness of sidewalks; the inadequate provision of pedestrian crossings and poor road and pavements user behavior. An urban design alternative for future pedestrian improvement in the area of study is set according to the paper's findings, while maintaining a distinct sense of Egyptian urbanity. This shows that the town planner can use various strategies to change the traffic composition in order to achieve better environments. Key words: Pedestrians, promenade, Boulevard, Cairo, Gameat El Dowal el Arabeya, shopping, sidewalk, quality of life and walking. -

Pizza Market Egypt Overvieww
Pizza Market Overview Egypt Pillars consultancy www.PILLARS-EG.COM [email protected] Table of contents • Pizza - Italian Restaurants Classification/ Products Offered • Pizza – Italian Outlets Egypt Nation-Wide (1/3) : • Review to Pizza Local Market – Pizza Hut – Domino’s Pizza – PAPA John’s – Little Caesars – Pizza King – Peppes Pizza – Cortigiano – Roma Pizza 2 Go – Pizza Plus • Other Pizza Outlets Online Ordering / Others 2 Pizza & Italian Restaurants Table of Contents : Pizza Hut Sbarro Papa Johns Little Caesars Pizza Roma Domino’s Pizza Pizza King Peppes Pizza Cortigiano La Casetta Majesty 3 Pizza - Italian Restaurants Classification/ Products Offered Local Local Italian Restaurant Pizza Chains Local Offer/ International Local Chains * Pizza/ Oriental Pizza + Type Pizza Chains Pizza Chains + Individual Restaurants Fast Food (Feteer) Avg. Class Class A/B A+B A+B B B+C Example Pizza Hut / Little Cesares/sbarro Pizza Y Y Y Y Italian Food Y Y Y Y Oriental Pizza - - - - Fast Food - - Y Y Others Y Y Pizza Hut Thomas Lacasetta Pizza King Majesty Papa John’s Tabasco Cortigiano/ Pizza Plus Dawwar/ Example Sbarro Pizza Pomodoro/Condetti/ Cook Door Pepes Pizza Little Caesars Not Much preferred as Much Preferred for Class outing for class A as A as perceived as outlets Comments preference towards Local of the choice for Pizza Italian Chains High Average Check / higher than Average 4 Pizza – Italian Outlets Egypt Nation-Wide (1/3) : Int’l Local Other “On-line” Chains Chains* Chains & Outlets La Rosa 19 Road, Degla, Maadi shahiya.com La Casetta