This Item Is the Archived Peer-Reviewed Author-Version Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Description of Two New Ecuadorian Zilchistrophia Weyrauch 1960
A peer-reviewed open-access journal ZooKeys 453: 1–17 (2014)Description of two new Ecuadorian Zilchistrophia Weyrauch 1960... 1 doi: 10.3897/zookeys.453.8605 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Description of two new Ecuadorian Zilchistrophia Weyrauch, 1960, with the clarification of the systematic position of the genus based on anatomical data (Gastropoda, Stylommatophora, Scolodontidae) Barna Páll-Gergely1, Takahiro Asami1 1 Department of Biology, Shinshu University, Matsumoto 390-8621, Japan Corresponding author: Barna Páll-Gergely ([email protected]) Academic editor: M. Haase | Received 17 September 2014 | Accepted 14 October 2014 | Published 10 November 2014 http://zoobank.org/741A5972-D4B3-46E9-A5CA-8F38A2E90B5B Citation: Páll-Gergely B, Asami T (2014) Description of two new Ecuadorian Zilchistrophia Weyrauch, 1960, with the clarification of the systematic position of the genus based on anatomical data (Gastropoda, Stylommatophora, Scolodontidae). ZooKeys 453: 1–17. doi: 10.3897/zookeys.453.8605 Abstract Two new species of the genus Zilchistrophia Weyrauch, 1960 are described from Eastern Ecuadorian rain forest: Zilchistrophia hilaryae sp. n. and Z. shiwiarorum sp. n. These two new species extend the distribu- tion of the genus considerably northwards, because congeners have been reported from Peru only. For the first time we present anatomical data (radula, buccal mass, morphology of the foot and the genital struc- ture) of Zilchistrophia species. According to these, the genus belongs to the family Scolodontidae, sub- family Scolodontinae (=“Systrophiini”). The previously assumed systematic relationship of Zilchistrophia with the Asian Corillidae and Plectopylidae based on the similarly looking palatal plicae is not supported. Keywords Systrophiidae, Plectopylidae, Plectopylis, Corillidae, anatomy, taxonomy Copyright Barna Páll-Gergely, Takahiro Asami. -

The Malacological Society of London
ACKNOWLEDGMENTS This meeting was made possible due to generous contributions from the following individuals and organizations: Unitas Malacologica The program committee: The American Malacological Society Lynn Bonomo, Samantha Donohoo, The Western Society of Malacologists Kelly Larkin, Emily Otstott, Lisa Paggeot David and Dixie Lindberg California Academy of Sciences Andrew Jepsen, Nick Colin The Company of Biologists. Robert Sussman, Allan Tina The American Genetics Association. Meg Burke, Katherine Piatek The Malacological Society of London The organizing committee: Pat Krug, David Lindberg, Julia Sigwart and Ellen Strong THE MALACOLOGICAL SOCIETY OF LONDON 1 SCHEDULE SUNDAY 11 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 2:00-6:00 pm Registration - Merrill Hall 10:30 am-12:00 pm Unitas Malacologica Council Meeting - Merrill Hall 1:30-3:30 pm Western Society of Malacologists Council Meeting Merrill Hall 3:30-5:30 American Malacological Society Council Meeting Merrill Hall MONDAY 12 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 7:30-8:30 am Breakfast - Crocker Dining Hall 8:30-11:30 Registration - Merrill Hall 8:30 am Welcome and Opening Session –Terry Gosliner - Merrill Hall Plenary Session: The Future of Molluscan Research - Merrill Hall 9:00 am - Genomics and the Future of Tropical Marine Ecosystems - Mónica Medina, Pennsylvania State University 9:45 am - Our New Understanding of Dead-shell Assemblages: A Powerful Tool for Deciphering Human Impacts - Sue Kidwell, University of Chicago 2 10:30-10:45 -

Number 59 August 2012
Number 59 (August 2012) The Malacologist Page 1 NUMBER 59 AUGUST 2012 Contents AGM CONFERENCE—MOLLUSCAN LIFE HISTORIES EDITORIAL …………………………….. ..........................2 Programme......................................................................................14 RESEARCH GRANT REPORTS: Abstracts ........................................................................................15 Species diversification in rainforest land snails ANNUAL GENERAL MEETING Dinarzarde Raheem.......................................................................3 Annual Report of Council ...................................................27 Heat shock and gene-repositioning in in Biomphalaria glabrata OBITUARY Halim Arica.................................……...…....... ...........................4 Dr Anthony South ...............................................................32 Assessing effects of dams on population genetic structure of Leptodea BOOK REVIEWS fragilis and its larval host. Sea snails on rocky shores—John Crothers.........................33 Sheila Langosch & Mary V. Ashley..............................................6 Never eat blue food—Malcolm Rigby.................................33 Living with acidification: the effects of rising seawater pCO on 2 Moluscos Marinos de Andalucía juvenile development in the common whelk Buccinum undatum Serge Gofas, Diego Moreno & Carmen Salas (eds) ......34 Kathryn E. Smith ...........................................................................8 ORTHCOMING EETINGS ANNUAL AWARD F M …………………………….…. -
Review of the Genus Endothyrella Zilch, 1960 with Description of Five
A peer-reviewed open-access journal ZooKeys 529:Review 1–70 (2015) of the genus Endothyrella Zilch, 1960 with description of five new species... 1 doi: 10.3897/zookeys.529.6139 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Review of the genus Endothyrella Zilch, 1960 with description of five new species (Gastropoda, Pulmonata, Plectopylidae) Barna Páll-Gergely1, Prem B. Budha2,5, Fred Naggs3, Thierry Backeljau4,5, Takahiro Asami1 1 Department of Biology, Shinshu University, Matsumoto 390-8621, Japan 2 Central Department of Zoology, Tribhuvan University, Kirtipur, Kathmandu, Nepal 3 Natural History Museum, Cromwell Road London, SW7 5BD, UK 4 Royal Belgian Institute of Natural Sciences, Vautierstraat 29, B-1000 Brussels, Belgium 5 University of Antwerp, Evolutionary Ecology Group Groenenborgerlaan 171, B-2020 Antwerp, Belgium Corresponding author: Barna Páll-Gergely ([email protected]) Academic editor: M. Haase | Received 24 July 2015 | Accepted 7 October 2015 | Published 26 October 2015 http://zoobank.org/AD4323B4-913C-447A-88A7-CE05EC8862A3 Citation: Páll-Gergely B, Budha PB, Naggs F, Backeljau T, Asami T (2015) Review of the genus Endothyrella Zilch, 1960 with description of five new species (Gastropoda, Pulmonata, Plectopylidae). ZooKeys 529: 1–70.doi: 10.3897/ zookeys.529.6139 Abstract All known taxa of the genus Endothyrella Zilch, 1960 (family Plectopylidae) are reviewed. Altogether 23 Endothyrella species are recognized. All species are illustrated and whenever possible, photographs of the available type specimens are provided. Five new species are described: E. angulata Budha & Páll-Gergely, sp. n., E. dolakhaensis Budha & Páll-Gergely, sp. n. and E. nepalica Budha & Páll-Gergely, sp. -
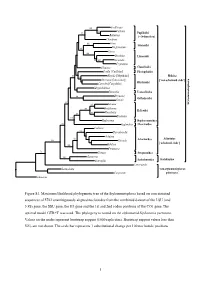
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Checklist of Terrestrial Gastropods of Kerala, India
Rec. zool. Surv. India: 105 (Part 1-2) : 73-81, 2005 CHECKLIST OF TERRESTRIAL GASTROPODS OF KERALA, INDIA RAJENDRA, G. MAVINKURVE, SANDHYA, P. SHANBHAG AND N. A. MADHYASTHA* Malacology Centre, Poornaprajna College, Udupi-576 101, Karnataka, India E-mail: [email protected];[email protected];[email protected] INTRODUCTION Leaf litter and soil form an arena for diverse interactions in tropical rain forests. It harbours many minute organisms, which play an important role in nutrient recycling. Molluscs belong to this ecological niche forming the epigeic species, which live and feed on the soil surface. Unfortunately, for the last 75 years or so, in South India, no attention has been paid for this important group with exceptions of some localized reviews and publications (Satyamurthy, 1960; Tonapi and Mulhekar, 1963; Tonapi 1971; Subba Rao and Mitra, 1979, 1986; Madhyastha et al., 2003, 2005; Mavinkurve et al., 2004b, in press; Sandhya et al., submitted). This report is in continuation of the list of the land snails present in the southern states that is being formulated .(Mavinkurve et al., 2004a) so that it will be helpful for any worker in the future to file new reports and carry further ecological studies. KERALA STATE Kerala described, as gods own country having an area of 38,863 sq km is tucked away in the southwest corner of India. It is geographically divided into the· highlands, midlands and lowlands. The highlands slope down from the Western Ghats, which rise to an average height of 900 m, with a number of peaks well over 1,800 m in height. -

Land Snails As Models for Biodiversity Assessment in Sri Lanka Annual
Land Snails as Models for Biodiversity Assessment in Sri Lanka Annual Report March 2005 Darwin Initiative for the Survival of Species Annual Report 1. Darwin Project Information Project Ref. Number E1 DPO 1 Project Title Land snails as models for biodiversity assessment in Sri Lanka Country Sri Lanka UK Contractor The Natural History Museum (NHM) Partner Organisation Wildlife Heritage Trust of Sri Lanka (WHT) Darwin Grant Value £68,500 Start/End dates 1.12.2003-30.11.2005 Reporting period 1.4.2004 – 31.3.2005 Report number 3 Authors, date Fred Naggs & Dinarzarde Raheem 20.5.2005 2. Project background The project seeks to build on the success of the 1999-2002 project Land snail diversity in Sri Lanka by conducting taxonomic studies as a basis for understanding the origins and dynamics of the fauna and establishing conservation strategy. 3. Project purpose and outputs • Taxonomic and systematic revisions with descriptions of new species • Advanced training and research experience for Dinarzarde Raheem, the 1999-2002 Sri Lankan project manager. • Training and work experience in electronic media communication for Hasantha Sanjeewa. • At least five research papers on the following subject areas: taxonomy and systematics, distribution and conservation. • Expansion of the interactive CD-ROM guide and publication of a new edition. • A new field guide structured to show species associated with different habitat types and including pest and exotic species. • Provision of an IUCN Red List evaluation of the Sri Lankan land snails. • An assessment of the distribution of land snail diversity in Sri Lanka and of key areas for conservation. Our projected timetable anticipated that the project could start at the beginning of October but the grant approval process resulted in a December 2003 start date when Fred Naggs was away on fieldwork and he could not commence work on the project until January 2004. -

Final Report
SPECIES DIVERSIFICATION IN A TROPICAL RAINFOREST LAND-SNAIL FAUNA Final Report DINARZARDE RAHEEM BELSPO Research Fellow (Non-EU Postdoc) Royal Belgian Institute of Natural Sciences, Vautierstraat 29, B-1000, Brussels 1. BACKGROUND Terrestrial biological diversity is not distributed evenly across the globe, but is concentrated in the forested tropics (Myers et al., 2000; Sechrest et al., 2002; Mittermeier et al., 2004). Our understanding of the evolutionary processes that have generated and maintained this diversity is poor, but such knowledge will be vital for conserving both the patterns and processes of diversity in the face of ongoing and future environmental change (Mace et al., 2003; Moritz & McDonald, 2005). Using land snails as a focal group and an approach centred on molecular systematics and community ecology, my 18-month fellowship aimed to investigate speciation and ecological diversification in two Sri Lankan rainforest snail genera, the pulmonate Corilla and the caenogastropod Theobaldius. In common with other taxa from other tropical rainforest regions (Moritz, et al., 2000; Hall, 2005; Weir, 2006; Brumfield & Edwards, 2007; Elias et al., 2009; Santos et al., 2009), Corilla and Theobaldius are represented by distinct species assemblages in lowland and montane rainforest. The study had two broad objectives: first, to reconstruct the evolution of these distinct lowland and montane assemblages, and second, to explore the relationship between species diversification and niche evolution. Work to date has been focussed on carrying out molecular and initial phylogenetic analyses on the two land-snail genera, Corilla and Theobaldius. In addition, I have been working on a major new taxonomic revision (Raheem et al., in press) on the land-snail fauna of the Western Ghats of India, as well two smaller related projects, one on the widespread Asian land snail Macrochlamys, and the other on the type collections of William Benson, the pioneering 19th century worker on Indian land snails. -

Exploring the Shell-Based Taxonomy of the Sri Lankan Land Snail Corilla H
Accepted Manuscript Exploring the shell-based taxonomy of the Sri Lankan land snail Corilla H. and A. Adams, 1855 (Pulmonata: Corillidae) using mitochondrial DNA D.C. Raheem, K. Breugelmans, C.M. Wade, F.C. Naggs, T. Backeljau PII: S1055-7903(16)30443-2 DOI: http://dx.doi.org/10.1016/j.ympev.2016.12.020 Reference: YMPEV 5699 To appear in: Molecular Phylogenetics and Evolution Received Date: 5 August 2016 Revised Date: 8 December 2016 Accepted Date: 20 December 2016 Please cite this article as: Raheem, D.C., Breugelmans, K., Wade, C.M., Naggs, F.C., Backeljau, T., Exploring the shell-based taxonomy of the Sri Lankan land snail Corilla H. and A. Adams, 1855 (Pulmonata: Corillidae) using mitochondrial DNA, Molecular Phylogenetics and Evolution (2016), doi: http://dx.doi.org/10.1016/j.ympev. 2016.12.020 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Exploring the shell-based taxonomy of the Sri Lankan land snail Corilla H. and A. Adams, 1855 (Pulmonata: Corillidae) using mitochondrial DNA D.C. Raheema,b,*, K. Breugelmansa, C.M. Wadec, F.C. Naggsb, T. Backeljaua,d a. Royal Belgian Institute of Natural Sciences, Vautierstraat 29, B-1000 Brussels, Belgium b. -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-205 Poster Presentations…………………………………………………………………………………207-270 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity. -

2007 Red List of Threatened Fauna and Flora of Sri Lanka
The 2007 Red List of Threatened Fauna and Flora of Sri Lanka This publication has been jointly prepared by The World Conservation Union (IUCN) in Sri Lanka and the Ministry of Environment and Natural Resources. The preparation and printing of this document was carried out with the financial assistance of the Protected Area Management and Wildlife Conservation Project and Royal Netherlands Embassy in Sri Lanka. i The designation of geographical entities in this book, and the presentation of the material do not imply the expression of any opinion whatsoever on the part of The World Conservation Union (IUCN) and Ministry of Environment and Natural Resources concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of The World Conservation Union (IUCN) and Ministry of Environment and Natural Resources. This publication has been jointly prepared by The World Conservation Union (IUCN) Sri Lanka and the Ministry of Environment and Natural Resources. The preparation and publication of this document was undertaken with financial assistance from the Protected Area Management and Wildlife Conservation Project and the Royal Netherlands Government. Published by: The World Conservation Union (IUCN) and Ministry of Environment and Natural Resources, Colombo, Sri Lanka. Copyright: © 2007, International Union for Conservation of Nature and Natural Resources and Ministry of Environment and Natural Resources, Sri Lanka. Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. -

Comparison of DNA Extraction Methods for Non-Marine Molluscs
bioRxiv preprint doi: https://doi.org/10.1101/863167; this version posted December 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Comparison of DNA extraction methods for non-marine molluscs: Is modified CTAB DNA 2 extraction method more efficient than DNA extraction kits? 3 4 Sudeshna Chakraborty, Anwesha Saha and Aravind N.A. 5 Center for Biodiversity and Conservation, 6 Ashoka Trust for Research in Ecology and the Environment (ATREE), 7 Royal Enclave, Sriramapura, Jakkur PO 8 Bangalore 560064, India 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 1 bioRxiv preprint doi: https://doi.org/10.1101/863167; this version posted December 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Abstract 2 Isolation of high molecular weight DNA from gastropod molluscs and its subsequent PCR 3 amplification is considered difficult due to excessive mucopolysaccharides secretion which co- 4 precipitate with DNA and obstruct successful amplification. In an attempt to address this issue, we 5 describe a modified CTAB DNA extraction method that proved to work significantly better with 6 a number of freshwater and terrestrial gastropod taxa.