Regulation of the Actin Binding Activity of Basic Calponin and Its Implication in Cytokinesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterizing Iqgap1-C Domain Binding to Phosphoinositides
CHARACTERIZING IQGAP1-C DOMAIN BINDING TO PHOSPHOINOSITIDES A Major Qualifying Project Report Submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE In partial fulfillment of the requirements for the Degree of Bachelor of Science in Biochemistry Written by: Approved by: __________________ . Christopher Dallarosa Dr. Arne Gericke Date: April 2018 Table of Contents Acknowledgments........................................................................................................................... 3 Abstract ........................................................................................................................................... 4 Introduction ..................................................................................................................................... 5 Background ..................................................................................................................................... 6 History ......................................................................................................................................... 6 Scaffolding Proteins .................................................................................................................... 6 IQGAP Protein Family ................................................................................................................ 8 IQGAPs in Yeast Cells ............................................................................................................ 8 Mammalian IQGAPs ............................................................................................................. -

Role of IQGAP1 in Carcinogenesis
cancers Review Role of IQGAP1 in Carcinogenesis Tao Wei and Paul F. Lambert * McArdle Laboratory for Cancer Research, Department of Oncology, University of Wisconsin School of Medicine and Public Health, Madison, WI 53705, USA; [email protected] * Correspondence: [email protected] Simple Summary: IQ motif-containing GTPase-activating protein 1 (IQGAP1) is a signal scaffolding protein that regulates a range of cellular activities by facilitating signal transduction in cells. IQGAP1 is involved in many cancer-related activities, such as proliferation, apoptosis, migration, invasion and metastases. In this article, we review the different pathways regulated by IQGAP1 during cancer development, and the role of IQGAP1 in different types of cancer, including cancers of the head and neck, breast, pancreas, liver, colorectal, stomach, and ovary. We also discuss IQGAP10s regulation of the immune system, which is of importance to cancer progression. This review highlights the significant roles of IQGAP1 in cancer and provides a rationale for pursuing IQGAP1 as a drug target for developing novel cancer therapies. Abstract: Scaffolding proteins can play important roles in cell signaling transduction. IQ motif- containing GTPase-activating protein 1 (IQGAP1) influences many cellular activities by scaffolding multiple key signaling pathways, including ones involved in carcinogenesis. Two decades of studies provide evidence that IQGAP1 plays an essential role in promoting cancer development. IQGAP1 is overexpressed in many types of cancer, and its overexpression in cancer is associated with lower survival of the cancer patient. Here, we provide a comprehensive review of the literature regarding the oncogenic roles of IQGAP1. We start by describing the major cancer-related signaling pathways Citation: Wei, T.; Lambert, P.F. -

A Study of Calponin's Role in Secretion
WORCESTER POLYTECHNIC INSTITUTE A Study of Calponin’s Role in Secretion The Proposed Model and the Experimental Design Christopher T. Rollins 4/30/2009 A Major Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for the Degree of Bachelor of Science in Biology and Biotechnology Approved: Jill Rulfs – Advisor Keywords: Actin, Cytoskeleton, Calponin, CaP, CN , Secretion , Exocytosis 1 A Study of Calponin’s R ole in Secretion Table of Contents Abstract .................................................................................................................................................. 2 Background ............................................................................................................................................. 2 Introduction ............................................................................................................................................ 6 Secretion Study – Model, Hypothesis, and Experiments ................................................................... 7 Materials and Methods ........................................................................................................................ 11 Amplification of pET, pCMV-HA, and GFP-Calponin Vectors ........................................................... 11 Creation of DH5α and XL-10 Gold Calcium Competent Cells ........................................................... 11 Transformation of Calcium Competent Cells .................................................................................. -
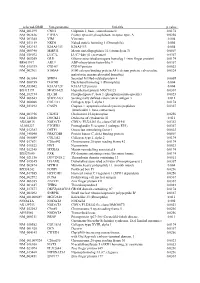
Supplementary Figures S1-S3
selected-GBID Uni-genename Uni-title p value NM_001299 CNN1 Calponin 1, basic, smooth muscle 0.0174 NM_002836 PTPRA Protein tyrosine phosphatase, receptor type, A 0.0256 NM_003380 VIM Vimentin 0.004 NM_033119 NKD1 Naked cuticle homolog 1 (Drosophila) 0.004 NM_052913 KIAA1913 KIAA1913 0.004 NM_005940 MMP11 Matrix metallopeptidase 11 (stromelysin 3) 0.0069 NM_018032 LUC7L LUC7-like (S. cerevisiae) 0.0367 NM_005269 GLI1 Glioma-associated oncogene homolog 1 (zinc finger protein) 0.0174 BE463997 ARL9 ADP-ribosylation factor-like 9 0.0367 NM_015939 CGI-09 CGI-09 protein 0.0023 NM_002961 S100A4 S100 calcium binding protein A4 (calcium protein, calvasculin, 0.0324 metastasin, murine placental homolog) NM_003014 SFRP4 Secreted frizzled-related protein 4 0.0005 NM_080759 DACH1 Dachshund homolog 1 (Drosophila) 0.004 NM_053042 KIAA1729 KIAA1729 protein 0.004 BX415194 MGC16121 Hypothetical protein MGC16121 0.0367 NM_182734 PLCB1 Phospholipase C, beta 1 (phosphoinositide-specific) 0.0023 NM_006643 SDCCAG3 Serologically defined colon cancer antigen 3 0.011 NM_000088 COL1A1 Collagen, type I, alpha 1 0.0174 NM_033292 CASP1 Caspase 1, apoptosis-related cysteine peptidase 0.0367 (interleukin 1, beta, convertase) NM_003956 CH25H Cholesterol 25-hydroxylase 0.0256 NM_144658 DOCK11 Dedicator of cytokinesis 11 0.011 AK024935 NODATA CDNA: FLJ21283 fis, clone COL01910 0.0363 AL050227 PTGER3 Prostaglandin E receptor 3 (subtype EP3) 0.0367 NM_012383 OSTF1 Osteoclast stimulating factor 1 0.0023 NM_145040 PRKCDBP Protein kinase C, delta binding protein 0.0069 NM_000089 -

RA0101-C.5-IFU-RUO Calponin-1 (Smooth Muscle Marker); Clone
Instructions For Use RA0 10 1-C.5 -IFU -RUO Revision: 1 Rev. Date: Oct. 14, 2014 Page 1 of 2 P.O. Box 3286 - Logan, Utah 84323, U.S.A. - Tel. (800) 729-8350 – Tel. (435) 755-9848 - Fax (435) 755-0015 - www.scytek.com Calponin-1 (Smooth Muscle Marker); Clone CNN1/832 & CALP (Concentrate) Availability/Contents: Item # Volume RA0101-C.5 0.5 ml Description: Species: Mouse Immunogen: Recombinant human CNN1 protein (CNN1/832); Crude human uterus extract (CALP) Clone: CNN1/832 & CALP Isotype: IgG1, kappa (CNN1/832); IgG1, kappa (CALP) Entrez Gene ID: 1264 (Human); 65204 (Rat) Hu Chromosome Loc.: 19p13.2 Synonyms: Calponin 1 basic smooth muscle; Calponin H1 smooth muscle; Calponin-1; CNN1; Cnn1; Sm Calp; SMCC Mol. Weight of Antigen: 34kDa Format: 200µg/ml of Ab purified from Bioreactor Concentrate by Protein A/G. Prepared in 10mM PBS with 0.05% BSA & 0.05% azide. Specificity: In Western blotting, this antibody reacts with only the 34kDa form of calponin in extracts of human aortic medial smooth muscle and is unreactive with fibroblast extracts of cultivated human foreskin. Background: Multiple isoelectric variants of calponin have been identified, however only two molecular weight isoforms exist; a 34kDa form and a 29kDa form. Expression of the 29kDa form, I-calponin, is primarily restricted to muscle of the urogenital tract, whereas the higher molecular weight variant has been demonstrated in vascular and visceral smooth muscle. Calponin is a calmodulin, F-actin and tropomyosin binding protein, which is thought to be involved in the regulation of smooth muscle contraction. -

Calponin 1 (CALP): Sc-58707
SANTA CRUZ BIOTECHNOLOGY, INC. Calponin 1 (CALP): sc-58707 BACKGROUND STORAGE Calponin regulates smooth muscle cell contraction and is a marker of smooth Store at 4° C, **DO NOT FREEZE**. Stable for one year from the date of muscle cell differentiation. Calponin, an Actin- and tropomyosin-binding pro- shipment. Non-hazardous. No MSDS required. tein, is characterized as an inhibitory factor of smooth-muscle actomyosin activity. Calponin is implicated in the regulation of smooth muscle contraction DATA through its interaction with F-Actin and inhibition of the Actin-activated MgATPase activity of phosphorylated myosin. Both properties are lost follow- ABC ing phosphorylation (primarily at Serine 175) by protein kinase C or calmodulin- 55 K – dependent protein kinase II. The three forms of Calponin, Calponin 1 (basic Calponin), Calponin 2 (neutral Calponin) and Calponin 3 (acidic Calponin) are 43 K – found in smooth muscle tissue. Additionally, Calponin 2 is found in heart < Calponin 1 muscle tissue and Calponin 3 is found in the brain. 34 K – CHROMOSOMAL LOCATION Calponin 1 (CALP) Alexa Fluor® 647: sc-58707 AF647. Calponin 1 (CALP): sc-58707. Immunoperoxidase Genetic locus: CNN1 (human) mapping to 19p13.2; Cnn1 (mouse) mapping Direct fluorescent western blot analysis of Calponin 1 staining of formalin fixed, paraffin-embedded human to 9 A3. expression in human smooth muscle (A) and human smooth muscle tissue showing cytoplasmic staining colon (B) tissue extracts and A-10 whole cell lysate (C). of smooth muscle cells. Blocked with UltraCruz® Blocking Reagent: sc-516214. SOURCE Calponin 1 (CALP) is a mouse monoclonal antibody raised against crude SELECT PRODUCT CITATIONS uterus extract of human origin. -

IQGAP1 Is a Phosphoinositide Effector and Kinase Scaffold. Adv Enzyme
Advances in Biological Regulation 60 (2016) 29e35 Contents lists available at ScienceDirect Advances in Biological Regulation journal homepage: www.elsevier.com/locate/jbior IQGAP1 is a phosphoinositide effector and kinase scaffold * Suyong Choi, Richard A. Anderson University of Wisconsin-Madison, School of Medicine and Public Health, 1300 University Avenue, Madison, WI 53706, USA article info abstract Article history: Phosphatidylinositol 4,5-bisphosphate (PI4,5P2) is a lipid messenger that regulates a wide Received 2 October 2015 variety of cellular functions. The majority of cellular PI4,5P2 is generated by isoforms of the Received in revised form 5 October 2015 type I phosphatidylinositol phosphate kinases (PIPKI) that are generated from three genes, Accepted 6 October 2015 and each PIPKI isoform has a unique distribution and function in cells. It has been shown Available online 28 October 2015 that the signaling specificity of PI4,5P2 can be determined by a physical association of PIPKs with PI4,5P2 effectors. IQGAP1 is newly identified as an interactor of multiple iso- Keywords: forms of PIPKs. Considering the versatile roles of IQGAP1 in cellular signaling pathways, Phosphoinositide fi Phosphatidylinositol phosphate kinase IQGAP1 may confer isoform-speci c roles of PIPKs in distinct cellular locations. In this mini IQGAP review, the emerging roles of PIPKs that are regulated by an association with IQGAP1 will Cell signaling be summarized. Focuses will be on cell migration, vesicle trafficking, cell signaling, and nuclear events. © 2015 Elsevier Ltd. All rights reserved. Contents 1. Introduction . .......................29 2. Phosphatidylinositol phosphate kinase interaction with IQGAP1 . .......................30 3. IQGAP1 interaction with PIPKIg regulates cell motility . -

Functional Gene Analysis of Resistance QTL Towards Phytophthora Sojae on Soybean
Functional Gene Analysis of Resistance QTL towards Phytophthora sojae on Soybean Chromosome 19 Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Anna K. Stasko Graduate Program in Plant Pathology The Ohio State University 2018 Dissertation Committee Anne E. Dorrance, Advisor Joshua J. Blakeslee Leah K. McHale Christopher G. Taylor Feng Qu Copyrighted by Anna Kathryn Stasko 2018 Abstract Phytophthora sojae is the causal agent of Phytophthora root and stem rot of soybean. One of the most effective disease management strategies against this pathogen is the use of resistant cultivars, primarily through single gene, Rps-mediated resistance. However, numerous populations of P. sojae have adapted to most Rps genes that are deployed in modern soybean cultivars, rendering them susceptible to this pathogen. Quantitative resistance, conferred by quantitative disease resistance loci (QDRL), offers an alternative to Rps-based resistance. Previous studies mapped two QDRL to chromosome 19 in the soybean cultivar Conrad, which has a high level of quantitative resistance. A recombinant inbred line (RIL) population derived from a cross of Conrad by Sloan (a moderately susceptible cultivar) used for mapping these QDRL was advanced to the F9:11 generation. This population was used to map/re-map the QDRL towards three isolates of P. sojae, and one isolate each of Pythium irregulare and Fusarium graminearum, using the SoySNP6K BeadChip for high-density marker genotyping. A total of ten, two, and three QDRL and suggestive QDRL were found that confer resistance to P. sojae, Py. irregulare, and F. -

Calponin and Cytoskeleton Dynamics in Macrophage Functions and the Ap Thogenesis of Atherosclerosis Rong Liu Wayne State University
Wayne State University Wayne State University Dissertations 1-1-2016 Calponin And Cytoskeleton Dynamics In Macrophage Functions And The aP thogenesis Of Atherosclerosis Rong Liu Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Part of the Immunology and Infectious Disease Commons, Medicine and Health Sciences Commons, and the Physiology Commons Recommended Citation Liu, Rong, "Calponin And Cytoskeleton Dynamics In Macrophage Functions And The aP thogenesis Of Atherosclerosis" (2016). Wayne State University Dissertations. 1650. http://digitalcommons.wayne.edu/oa_dissertations/1650 This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. CALPONIN AND CYTOSKELETON DYNAMICS IN MACROPHAGE FUNCTIONS AND THE PATHOGENESIS OF ATHEROSCLEROSIS by RONG LIU DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfilment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2016 MAJOR: PHYSIOLOGY Approved By: Advisor Date © COPYRIGHT BY RONG LIU 2016 All Rights ReserveD DEDICATION To my departed loving father for your guidance and encouragement during my youth and career development; to my dear mother for your love and support. ii ACKNOWLEDGEMENTS I am grateful to my dissertation advisor, Dr. J.-P. Jin. Your training and insights have been invaluable to me. Thank you for your tireless teaching and guidance. I sincerely thank the members of my dissertation committee, Dr. Steven Cala, Dr. Robert Lasley, Dr. Takeshi Sakamoto, Dr. Assia Shisheva and Dr. Jianjun Wang, for their support and advice. -

The Campylobacter Jejuni Ciad Effector Co-Opts the Host Cell Protein IQGAP1 to Promote Cell Entry
ARTICLE https://doi.org/10.1038/s41467-021-21579-5 OPEN The Campylobacter jejuni CiaD effector co-opts the host cell protein IQGAP1 to promote cell entry Nicholas M. Negretti 1, Christopher R. Gourley1, Prabhat K. Talukdar 1, Geremy Clair 2, ✉ Courtney M. Klappenbach 1, Cody J. Lauritsen1, Joshua N. Adkins 2 & Michael E. Konkel 1 Campylobacter jejuni is a foodborne pathogen that binds to and invades the epithelial cells lining the human intestinal tract. Maximal invasion of host cells by C. jejuni requires cell 1234567890():,; binding as well as delivery of the Cia proteins (Campylobacter invasion antigens) to the host cell cytosol via the flagellum. Here, we show that CiaD binds to the host cell protein IQGAP1 (a Ras GTPase-activating-like protein), thus displacing RacGAP1 from the IQGAP1 complex. This, in turn, leads to the unconstrained activity of the small GTPase Rac1, which is known to have roles in actin reorganization and internalization of C. jejuni. Our results represent the identification of a host cell protein targeted by a flagellar secreted effector protein and demonstrate that C. jejuni-stimulated Rac signaling is dependent on IQGAP1. 1 School of Molecular Biosciences, College of Veterinary Medicine, Washington State University, Pullman, WA, USA. 2 Integrative Omics, Pacific Northwest ✉ National Laboratory, Richland, WA, USA. email: [email protected] NATURE COMMUNICATIONS | (2021) 12:1339 | https://doi.org/10.1038/s41467-021-21579-5 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-21579-5 common strategy for disease-causing microbes is to complex known as the focal adhesion, a structure that links the manipulate the behavior and function of host cells within extracellular environment to the actin cytoskeleton, which is A 1 26,27 infected tissues .