Antiproliferative Activities of Alkaloids from Crinum Abyscinicum Hochst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Boophone Disticha
Micropropagation and pharmacological evaluation of Boophone disticha Lee Cheesman Submitted in fulfilment of the academic requirements for the degree of Doctor of Philosophy Research Centre for Plant Growth and Development School of Life Sciences University of KwaZulu-Natal, Pietermaritzburg April 2013 COLLEGE OF AGRICULTURE, ENGINEERING AND SCIENCES DECLARATION 1 – PLAGIARISM I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research contained in this thesis, except where otherwise indicated, is my original research. 2. This thesis has not been submitted for any degree or examination at any other University. 3. This thesis does not contain other persons’ data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. 4. This thesis does not contain other persons’ writing, unless specifically acknowledged as being sourced from other researchers. Where other written sources have been quoted, then: a. Their words have been re-written but the general information attributed to them has been referenced. b. Where their exact words have been used, then their writing has been placed in italics and inside quotation marks, and referenced. 5. This thesis does not contain text, graphics or tables copied and pasted from the internet, unless specifically acknowledged, and the source being detailed in the thesis and in the reference section. Signed at………………………………....on the.....….. day of ……......……….2013 ______________________________ SIGNATURE i STUDENT DECLARATION Micropropagation and pharmacological evaluation of Boophone disticha I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research reported in this dissertation, except where otherwise indicated is the result of my own endeavours in the Research Centre for Plant Growth and Development, School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg. -

Download Download
Vol. 1 No. 1 Natural Products and Biotechnology pp. 38-48 (2021) Determination of Toxic and Anthelmintic Activities of Ornithogalum nutans L., Sternbergia lutea (L.) Ker-Gawl. ex Spreng. and Allium stylosum O. Schwarz Mehmet Ozgur Atay1 , Buse Ardil1* , Mehlika Alper1 , Olcay Ceylan2 1 Department of Moleculer Biology and Genetics, Faculty of Science, Muğla Sıtkı Koçman University, Muğla, Turkey 2 Department of Biology Faculty of Science, Muğla Sıtkı Koçman University, Muğla, Turkey Article History Abstract Received : May 22, 2021 In this study, toxic and anthelmintic activities of methanol extracts of aerial and Revised : June 03, 2021 underground parts of Ornithogalum nutans L., Sternbergia lutea (L.) Ker-Gawl. ex Accepted : June 15, 2021 Spreng. and Allium stylosum O.Schwarz were investigated. In order to determine the anthelmintic activity, the time elapsed for the duration of paralysis and death was Keywords determined after the extracts of different concentrations (10, 20 and 30 mg/mL) were added to the Tubifex tubifex in petri dishes. Each concentration of A. stylosum aerial Ornithogalum nutans, and underground parts extracts showed high anthelmintic activity. In addition, aerial Sternbergia lutea, extract of O. nutans at a concentration of 30 mg/mL showed high anthelmintic Allium stylosum, activity. A. stylosum extracts showed a higher activity than the standard anthelmintic Artemia salina, drug. The toxic activity was determined against Artemia salina with brine shrimp Anthelmintic activity lethality test. Among all extracts, the underground extract of S. lutea showed the highest activity with 0.002 mg/mL, LC50, the aerial extract of O. nutans showed the lowest activity with 0.03 mg/mL, LC50. -

A Comparative Karyomorphological Analysis of Crinum Asiaticum L. and Crinum Latifolium L
ISSN (Online): 2349 -1183; ISSN (Print): 2349 -9265 TROPICAL PLANT RESEARCH 7(1): 51–54, 2020 The Journal of the Society for Tropical Plant Research DOI: 10.22271/tpr.2020.v7.i1.008 Research article A comparative karyomorphological analysis of Crinum asiaticum L. and Crinum latifolium L. from Paschim Medinipur district of West Bengal, India Anushree Dolai and Asis Kumar Nandi* Cytology and Molecular laboratory, Department of Botany and Forestry, Vidyasagar University, Midnapore, West Bengal, India *Corresponding Author: [email protected] [Accepted: 28 February 2020] Abstract: Crinum asiaticum and C. latifolium are two ornamental plant species with medicinal importance. These species have a host of biomolecules of pharmaceutical uses. The chromosomal study is a very basic one in characterizing the genetic material of a species. Earlier reports on such studies have shown both of 22 and 24 to represent the diploid number of chromosomes in the somatic cell of Crinum sp. The present study confirmed the 2n number as 22 for both of the species. However, these two species differ in respect of different parameters. Chromosome types are 10 metacentric and 12 submetacentric in C. asiaticum, while 10 metacentric, 6 submetacentric and 6 subterminal chromosomes in C. latifolium. Considerable variations are also evident in the total chromosomal length of the haploid set, symmetric index, degree of karyotype asymmetry, mean centromeric asymmetry, coefficient of variation of chromosome length, coefficient of variation of the centromeric index as well as the asymmetric index. These variations provide the chromosomal identity of these two species and also the nature of the relationship in them. Keywords: Chromosome study - Karyomorphology - Ideogram - Crinum species. -

STUDY on GROWTH, DEVELOPMENT and SOME BIOCHEMICAL ASPECTS of SEVERAL VARIETIES of Nerine
• STUDY ON GROWTH, DEVELOPMENT AND SOME BIOCHEMICAL ASPECTS OF SEVERAL VARIETIES OF Nerine by KUMALA DEWI partia,1 Submitted in,fulfilment of the requirements for the A degree of Master of Science Studies Department of Plant Science University of Tasmania May, 1993 DECLARATION To the best of my knowledge and belief, this thesis contains no material which has been submitted for the award of any other degree or diploma, nor does it contain any paraphrase of previously published material except where due reference is made in the text. Kumala Dewi ii ABSTRACT Nerine fothergillii bulbs were stored at different temperatures for a certain period of time and then planted and grown in an open condition. The effect of the different storage temperatures on carbohydrate -; content and endogenous gibberellins w Ct,5 examined in relation to flowering. Flowering percentage and flower number in each umbel was reduced when the bulbs were stored at 300 C while bulbs which received 50 C treatment possess earlier flowering and longer flower stalksthan bulbs without 5 0 C storage treatment. Carbohydrates in both outer and inner scales of N. fothergillii were examined semi-quantitatively by paper chromatography. Glucose, fructose and sucrose have been identified from paper chromatogramS. Endogenous gibberellins in N. fothergillii have been identified by GC - SIM and full mass spectra from GCMS. These include GA19, GA20 and G Al, their presence suggests the occurence of the early 13 - hydroxylation pathway. The response of N. bowdenii grown under Long Day (LD) and Short Day (SD) conditions w as studied. Ten plants from each treatment were examined at intervalsof 4 weeks. -

Complete Chloroplast Genomes Shed Light on Phylogenetic
www.nature.com/scientificreports OPEN Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae) Ju Namgung1,4, Hoang Dang Khoa Do1,2,4, Changkyun Kim1, Hyeok Jae Choi3 & Joo‑Hwan Kim1* Allioideae includes economically important bulb crops such as garlic, onion, leeks, and some ornamental plants in Amaryllidaceae. Here, we reported the complete chloroplast genome (cpDNA) sequences of 17 species of Allioideae, fve of Amaryllidoideae, and one of Agapanthoideae. These cpDNA sequences represent 80 protein‑coding, 30 tRNA, and four rRNA genes, and range from 151,808 to 159,998 bp in length. Loss and pseudogenization of multiple genes (i.e., rps2, infA, and rpl22) appear to have occurred multiple times during the evolution of Alloideae. Additionally, eight mutation hotspots, including rps15-ycf1, rps16-trnQ-UUG, petG-trnW-CCA , psbA upstream, rpl32- trnL-UAG , ycf1, rpl22, matK, and ndhF, were identifed in the studied Allium species. Additionally, we present the frst phylogenomic analysis among the four tribes of Allioideae based on 74 cpDNA coding regions of 21 species of Allioideae, fve species of Amaryllidoideae, one species of Agapanthoideae, and fve species representing selected members of Asparagales. Our molecular phylogenomic results strongly support the monophyly of Allioideae, which is sister to Amaryllioideae. Within Allioideae, Tulbaghieae was sister to Gilliesieae‑Leucocoryneae whereas Allieae was sister to the clade of Tulbaghieae‑ Gilliesieae‑Leucocoryneae. Molecular dating analyses revealed the crown age of Allioideae in the Eocene (40.1 mya) followed by diferentiation of Allieae in the early Miocene (21.3 mya). The split of Gilliesieae from Leucocoryneae was estimated at 16.5 mya. -

Atoll Research Bulletin No. 503 the Vascular Plants Of
ATOLL RESEARCH BULLETIN NO. 503 THE VASCULAR PLANTS OF MAJURO ATOLL, REPUBLIC OF THE MARSHALL ISLANDS BY NANCY VANDER VELDE ISSUED BY NATIONAL MUSEUM OF NATURAL HISTORY SMITHSONIAN INSTITUTION WASHINGTON, D.C., U.S.A. AUGUST 2003 Uliga Figure 1. Majuro Atoll THE VASCULAR PLANTS OF MAJURO ATOLL, REPUBLIC OF THE MARSHALL ISLANDS ABSTRACT Majuro Atoll has been a center of activity for the Marshall Islands since 1944 and is now the major population center and port of entry for the country. Previous to the accompanying study, no thorough documentation has been made of the vascular plants of Majuro Atoll. There were only reports that were either part of much larger discussions on the entire Micronesian region or the Marshall Islands as a whole, and were of a very limited scope. Previous reports by Fosberg, Sachet & Oliver (1979, 1982, 1987) presented only 115 vascular plants on Majuro Atoll. In this study, 563 vascular plants have been recorded on Majuro. INTRODUCTION The accompanying report presents a complete flora of Majuro Atoll, which has never been done before. It includes a listing of all species, notation as to origin (i.e. indigenous, aboriginal introduction, recent introduction), as well as the original range of each. The major synonyms are also listed. For almost all, English common names are presented. Marshallese names are given, where these were found, and spelled according to the current spelling system, aside from limitations in diacritic markings. A brief notation of location is given for many of the species. The entire list of 563 plants is provided to give the people a means of gaining a better understanding of the nature of the plants of Majuro Atoll. -
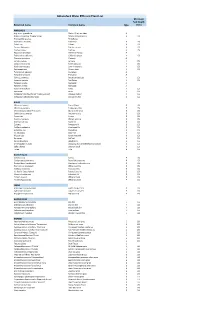
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical Name Common Name Type (Mm)
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical name Common name type (mm) ANNUALS Argemone grandifolia Statice/Sea Lavendar A Begonia x hybrida 'Dragon Wings' Dragon Wing begonia A 150 Bracteantha species Strawflower Calendula officinalis Calendula A 150 Coleus ssp. Coleus A 150 Cosmos bipinnatus Garden cosmos A 150 Cuphea llavea Cuphea A 150 Dyssocua tenuiloba Dahlberg Daisey A Eschscholzia californica California poppy A 150 Gazania spendens Gazania A Lantana camara Lantana A 150 Lobularia maritima Sweet alyssum A 150 Nigella damascena Love-in-the-mist A 150 Oesteopermum African daisy A 150 Pelargonium species Geranium A Portulaca oleracea Portulaca A Salvia guaranitica Anise-scented sage A 150 Scaevola aemula Fan flower A 150 Targetes erecta Marogold A Targetes erecta Marogold A Viola x wittrockiana Pansy A 150 Zinnia ssp. Zinnia A 150 Verbascum bombyciferum 'Arctic Summer' Broussa mullein A 150 Verbascum phlomoides 'Spica' Orange mullein A 150 BULBS Allium christophii Star of Persia B 150 Allium karataviense Turkestan onion B 150 Chionodoxa forbesii 'Pink Giant' Glory of the snow B 150 Colchicum autumnale Autumn crocus B 150 Crocus ssp. Crocus B 150 Eranthis hyemalis Winter aconite B 150 Erythronium ssp. Fawn lily B 150 Eucomis Pineapple lily B 150 Fritillaria meleagris Checkered lily B 150 Galanthus ssp. Snowdrop B 150 Iris reticulata Dwarf iris B 150 Muscari ssp. Grape hyacinth B 150 Narcissus Daffodil B 150 Nerine bowdenii Bowden lily B 150 Ornithogalum nutans Drooping Star of Bethlehem/Silverbells B 150 Scilla -

GROUP C: OTHER GROUND-DWELLING HERBS (Not Grasses Or Ferns)
Mangrove Guidebook for Southeast Asia Part 2: DESCRIPTIONS – Other ground-dwelling herbs GROUP C: OTHER GROUND-DWELLING HERBS (not grasses or ferns) 327 Mangrove Guidebook for Southeast Asia Part 2: DESCRIPTIONS – Other ground-dwelling herbs Fig. 52. Acanthus ebracteatus Vahl. (a) Habit, (b) bud, and (c) flower. 328 Mangrove Guidebook for Southeast Asia Part 2: DESCRIPTIONS – Other ground-dwelling herbs ACANTHACEAE 52 Acanthus ebracteatus Vahl. Synonyms : Unknown. Vernacular name(s) : Sea Holly (E), Jeruju (hitam) (Mal.), Jeruju (Ind.), Ô rô (Viet.), Trohjiekcragn pkapor sar, Trohjiekcragn slekweng (Camb.), Ngueak plaamo dok muang (Thai) Description : Acanthus ebracteatus resembles Acanthus ilicifolius (see next page), but all parts are smaller. Flowers measure 2-3 cm and are (usually) white; the fruit is shorter than 2.0 cm; seeds measure 5-7 mm. Flowers have only one main enveloping leaflet, as the secondary ones are usually rapidly shed. The species described by Rumphius as the male specimen of Acanthus ilicifolius was later identified by Merrill as Acanthus ebracteatus Vahl. Some authors regard Acanthus ebracteatus, Acanthus ilicifolius and Acanthus volubilis as one highly variable species (e.g. Heyne, 1950). Note that in Acanthus young leaves or leaves on the ends of branches may be unarmed (i.e. without spines), while older specimens may be armed. Ecology : Where this species occurs together with Acanthus ilicifolius the two seem distinct in the characters used in the descriptions, but they are often confused. Flowering usually occurs in June (in Indonesia). True mangrove species. Distribution : From India to tropical Australia, Southeast Asia and the west Pacific islands (e.g. Solomon Islands). -

TAXANOMY of the GENUS Crinum (Amaryllidaceae)
Cey. J. Sci. (Bio. Sci.) 35 (1): 53 -72, 2006 53 AN EMPIRICAL STUDY ON THE TAXONOMY OF CRINUM ZEYLANICUM (L.) L. AND CRINUM LATIFOLIUM L. (AMARYLLIDACEAE) OCCURRING IN SRI LANKA D.M.D. Yakandawala* and T.M. Samarakoon Department of Botany, Faculty of Science, University of Peradeniya, Peradeniya. Sri Lanka. Accepted 27 February 2006 ABSTRACT Crinum latifolium L. and C. zeylanicum (L.) L. are two Crinum species native to Sri Lanka, but their species delimitation has been a point of debate since their establishment as separate species. During the recent revision of the Sri Lankan Amaryllidaceae, both species have been recognized. The separation of the two species is based on the leaf undulation and the size of the leaves. Field experiences suggest the occurrence of Crinum species with other distinct characters, raising the question of their species limits. Therefore, a detailed taxonomic study on species limits of C. latifolium and C. zeylanicum was carried out to solve the taxonomic ambiguity, based on empirical methods. Specimens were collected from all possible geographical locations. Morphological characteristics with distinct character states were studied at both macroscopic and microscopic level and coded into data matrices. Species limits were determined by phenetic and phylogenetic methods. The results clearly suggested the occurrence of two morphologically distinct groups supporting the recognition of C. latifolium L. and C. zeylanicum (L.) L. Furthermore, two morphologically distinct forms of C. zeylanicum were identified as occurring in Sri Lanka which had not been previously recorded. In view of the fact that the characters of these two types are stable and not dependent on the environment, formal taxonomic ranks could be offered. -

David Domoneys Complete Guide to Seeds and Bulbs
Do David money’s COMPLETE GUIDE To bs Seeds and Bul Let’s begin at the beginning Knowing where plants come from and how they grow is important if you want to grow strong, healthy plants. Understanding seeds and bulbs will help you become a better gardener. What’s in this guide? First we will look at seeds and the different techniques for sowing them indoors and directly outside. We will also explore how to care for seedlings, plus the right ways to collect and store seeds from your plants. In the second part, we will talk about the different types of bulbs, how to plant them and the best ways to create year-round displays. Seeds Growing your own plants from seed is always exciting. Seeing them develop into new plants is very rewarding, and it’s a cost-effective way to add to your garden. What are seeds? A seed is the unit of reproduction for most plants. It contains all the genetic information needed to create an entirely new plant, plus a nutrient store to help it get started. So many types of plants can be grown from seed, from herbs and vegetables to annual and perennial flowering species. Most seeds need warmth and moisture to germinate, but some need bright light or a cooler environment to get started. Others require soaking before sowing. There will be full instructions on the seed packet, but here I will show you a few general tips to help you get started. Find more guides at davidomoney.com Sowing outdoors Some seeds can be sown directly into the ground where you want them to grow. -

ALKALOID-BEARING PLANTS and THEIR CONTAINED ALKALOIDS by J
ALKALOID-BEARING PLANTS and Their Contained Alkaloids TT'TBUCK \ \ '■'. Technical Bulletin No. 1234 AGRICULTURAL RESEARCH SERVICE U.S. DEPARTMENT OF AGRICULTURE ACKNOWLEDGMENTS The authors are indebted to J. W. Schermerhorn and M. W. Quimby, Massachusetts College of Pharmacy, for access to the original files of the Lynn Index; to K. F. Rauiïauf, Smith, Kline & French Labora- tories, and to J. H. Hoch, Medical College of South Carolina, for extensive lists of alkaloid plants; to V. S. Sokolov, V. L. Komarova Academy of Science, Leningrad, for a copy of his book; to J. M. Fogg, Jr., and H. T. Li, Morris Arboretum, for botanical help and identification of Chinese drug names ; to Michael Dymicky, formerly of the Eastern Utilization Research and Development Division, for ex- tensive translations; and to colleagues in many countries for answering questions raised during the compilation of these lists. CONTENTS Page Codes used in table 1 2 Table 1.—Plants and their contained alkaloids 7 Table 2.—Alkaloids and the plants in which they occur 240 Washington, D.C. Issued August 1961 For sale by the Superintendent of Documents, Qovemment Printing OflSce. Washington 25, D.C. Price $1 ALKALOID-BEARING PLANTS AND THEIR CONTAINED ALKALOIDS By J. J. WiLLAMAN, chemist, Eastern Utilization Research and Development Division, and BERNICE G. SCHUBERT, taxonomist. Crops Research Division, Agricultural Research Service This compilation assembles in one place all the scattered information on the occurrence of alkaloids in the plant world. It consists of two lists: (1) The names of the plants and of their contained alkaloids; and (2) the names and empirical formulas of the alkaloids.