Vietnam Pheasant (Lophura Edwardsi)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Sage-Grouse Hunting Season
CHAPTER 11 UPLAND GAME BIRD AND SMALL GAME HUNTING SEASONS Section 1. Authority. This regulation is promulgated by authority of Wyoming Statutes § 23-1-302 and § 23-2-105 (d). Section 2. Hunting Regulations. (a) Bag and Possession Limit. Only one (1) daily bag limit of each species of upland game birds and small game may be taken per day regardless of the number of hunt areas hunted in a single day. When hunting more than one (1) hunt area, a person’s daily and possession limits shall be equal to, but shall not exceed, the largest daily and possession limit prescribed for any one (1) of the specified hunt areas in which the hunting and possession occurs. (b) Evidence of sex and species shall remain naturally attached to the carcass of any upland game bird in the field and during transportation. For pheasant, this shall include the feathered head, feathered wing or foot. For all other upland game bird species, this shall include one fully feathered wing. (c) No person shall possess or use shot other than nontoxic shot for hunting game birds and small game with a shotgun on the Commission’s Table Mountain and Springer wildlife habitat management areas and on all national wildlife refuges open for hunting. (d) Required Clothing. Any person hunting pheasants within the boundaries of any Wyoming Game and Fish Commission Wildlife Habitat Management Area, or on Bureau of Reclamation Withdrawal lands bordering and including Glendo State Park, shall wear in a visible manner at least one (1) outer garment of fluorescent orange or fluorescent pink color which shall include a hat, shirt, jacket, coat, vest or sweater. -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

A Molecular Phylogeny of the Pheasants and Partridges Suggests That These Lineages Are Not Monophyletic R
Molecular Phylogenetics and Evolution Vol. 11, No. 1, February, pp. 38–54, 1999 Article ID mpev.1998.0562, available online at http://www.idealibrary.com on A Molecular Phylogeny of the Pheasants and Partridges Suggests That These Lineages Are Not Monophyletic R. T. Kimball,* E. L. Braun,*,† P. W. Zwartjes,* T. M. Crowe,‡,§ and J. D. Ligon* *Department of Biology, University of New Mexico, Albuquerque, New Mexico 87131; †National Center for Genome Resources, 1800 Old Pecos Trail, Santa Fe, New Mexico 87505; ‡Percy FitzPatrick Institute, University of Capetown, Rondebosch, 7700, South Africa; and §Department of Ornithology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024-5192 Received October 8, 1997; revised June 2, 1998 World partridges are smaller and widely distributed in Cytochrome b and D-loop nucleotide sequences were Asia, Africa, and Europe. Most partridge species are used to study patterns of molecular evolution and monochromatic and primarily dull colored. None exhib- phylogenetic relationships between the pheasants and its the extreme or highly specialized ornamentation the partridges, which are thought to form two closely characteristic of the pheasants. related monophyletic galliform lineages. Our analyses Although the order Galliformes is well defined, taxo- used 34 complete cytochrome b and 22 partial D-loop nomic relationships are less clear within the group sequences from the hypervariable domain I of the (Verheyen, 1956), due to the low variability in anatomi- D-loop, representing 20 pheasant species (15 genera) and 12 partridge species (5 genera). We performed cal and osteological traits (Blanchard, 1857, cited in parsimony, maximum likelihood, and distance analy- Verheyen, 1956; Lowe, 1938; Delacour, 1977). -

Scottish Birds 22: 9-19
Scottish Birds THE JOURNAL OF THE SOC Vol 22 No 1 June 2001 Roof and ground nesting Eurasian Oystercatchers in Aberdeen The contrasting status of Ring Ouzels in 2 areas of upper Deeside The distribution of Crested Tits in Scotland during the 1990s Western Capercaillie captures in snares Amendments to the Scottish List Scottish List: species and subspecies Breeding biology of Ring Ouzels in Glen Esk Scottish Birds The Journal of the Scottish Ornithologists' Club Editor: Dr S da Prato Assisted by: Dr I Bainbridge, Professor D Jenkins, Dr M Marquiss, Dr J B Nelson, and R Swann Business Editor: The Secretary sac, 21 Regent Terrace Edinburgh EH7 5BT (tel 0131-5566042, fax 0131 5589947, email [email protected]). Scottish Birds, the official journal of the Scottish Ornithologists' Club, publishes original material relating to ornithology in Scotland. Papers and notes should be sent to The Editor, Scottish Birds, 21 Regent Terrace, Edinburgh EH7 SBT. Two issues of Scottish Birds are published each year, in June and in December. Scottish Birds is issued free to members of the Scottish Ornithologists' Club, who also receive the quarterly newsletter Scottish Bird News, the annual Scottish Bird Report and the annual Raplor round up. These are available to Institutions at a subscription rate (1997) of £36. The Scottish Ornithologists' Club was formed in 1936 to encourage all aspects of ornithology in Scotland. It has local branches which meet in Aberdeen, Ayr, the Borders, Dumfries, Dundee, Edinburgh, Glasgow, Inverness, New Galloway, Orkney, St Andrews, Stirling, Stranraer and Thurso, each with its own programme of field meetings and winter lectures. -

Traffic.Org/Home/2015/12/4/ Thousands-Of-Birds-Seized-From-East-Java-Port.Html
TRAFFIC IN THE MARKET FOR EXTINCTION REPORT Eastern and Central Java AUGUST 2016 Serene C. L. Chng and James A. Eaton TRAFFIC Report: In The Market for Extinction: Eastern and Central Java 1 TRAFFIC REPORT TRAFFIC, the wild life trade monitoring net work, is the leading non-governmental organization working globally on trade in wild animals and plants in the context of both biodiversity conservation and sustainable development. TRAFFIC is a strategic alliance of WWF and IUCN. Reprod uction of material appearing in this report requires written permission from the publisher. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations con cern ing the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views of the authors expressed in this publication are those of the writers and do not necessarily reflect those of TRAFFIC, WWF or IUCN. Published by TRAFFIC. Southeast Asia Regional Office Unit 3-2, 1st Floor, Jalan SS23/11 Taman SEA, 47400 Petaling Jaya Selangor, Malaysia Telephone : (603) 7880 3940 Fax : (603) 7882 0171 Copyright of material published in this report is vested in TRAFFIC. © TRAFFIC 2016. ISBN no: 978-983-3393-50-3 UK Registered Charity No. 1076722. Suggested citation: Chng, S.C.L. and Eaton, J.A. (2016). In the Market for Extinction: Eastern and Central Java. TRAFFIC. Petaling Jaya, Selangor, Malaysia. Front cover photograph: An Oriental Bay Owl Phodilus badius displayed for sale at Malang Bird Market Credit: Heru Cahyono/TRAFFIC IN THE MARKET FOR EXTINCTION Eastern and Central Java Serene C. -
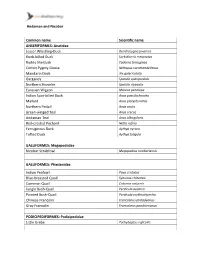
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

The Eco-Ethology of the Karoo Korhaan Eupodotis Virgorsil
THE ECO-ETHOLOGY OF THE KAROO KORHAAN EUPODOTIS VIGORSII. BY M.G.BOOBYER University of Cape Town SUBMITIED IN PARTIAL FULFILMENT OF THE DEGREE OF MASTER OF SCIENCE (ORNITHOLOGY) UNIVERSITY OF CAPE TOWN RONDEBOSCH 7700 CAPE TOWN The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town University of Cape Town PREFACE The study of the Karoo Korhaan allowed me a far broader insight in to the Karoo than would otherwise have been possible. The vast openness of the Karoo is a monotony to those who have not stopped and looked. Many people were instrumental in not only encouraging me to stop and look but also in teaching me to see. The farmers on whose land I worked are to be applauded for their unquestioning approval of my activities and general enthusiasm for studies concerning the veld and I am particularly grateful to Mnr. and Mev. Obermayer (Hebron/Merino), Mnr. and Mev. Steenkamp (Inverdoorn), Mnr. Bothma (Excelsior) and Mnr. Van der Merwe. Alwyn and Joan Pienaar of Bokvlei have my deepest gratitude for their generous hospitality and firm friendship. Richard and Sue Dean were a constant source of inspiration throughout the study and their diligence and enthusiasm in the field is an example to us all. -

Epidemiology of Influenza Virus H5n1 in Islamabad Capital Territory by Zahida Fatima (2005-Va-246) a Thesis Submitted in the Pa
EPIDEMIOLOGY OF INFLUENZA VIRUS H5N1 IN ISLAMABAD CAPITAL TERRITORY BY ZAHIDA FATIMA (2005-VA-246) A THESIS SUBMITTED IN THE PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN EPIDEMIOLOGY AND PUBLIC HEALTH UNIVERSITY OF VETERINARY & ANIMAL SCIENCES, LAHORE (2015) To The Controller of Examinations, University of Veterinary and Animal Sciences, Lahore. We, the supervisory committee, certify that the contents and form of the thesis, submitted by ZAHIDA FATIMA, Regd. No. 2005-VA-246 been found satisfactory and recommend that it be processed for the evaluation by the External Examiner(s) for the award of the degree. PROF. DR. MUHAMMAD ATHAR KHAN _______________________ SUPERVISOR DR. KHALID NAEEM _______________________ CO-SUPERVISOR PROF.DR. MANSOOR UD DIN AHMAD _______________________ MEMBER PROF.DR. KHUSI MUHAMMAD ______________________ MEMBER DEDICATED TO MY LATE FATHER MAY HIS SOUL REST IN BEST PEACE (AAMEEN) i () In the name of Allah the most magnificent and the most beneficent. All praise for ALLAH All Mighty who has the control and command of each and every thing. It is He who has sent down to you, [O Muhammad], the Book; in it are verses [that are] precise - they are the foundation of the Book - and others unspecific. As for those in whose hearts is deviation [from truth], they will follow that of it which is unspecific, seeking discord and seeking an interpretation [suitable to them]. And no one knows its [true] interpretation except Allah. But those firms in knowledge say, "We believe in it. All [of it] is from our Lord." And no one will be reminded except those of understanding. -

A Molecular Phylogeny of the Peacock-Pheasants (Galliformes: Polyplectron Spp.) Indicates Loss and Reduction of Ornamental Traits and Display Behaviours
Biological Journal of the Linnean Society (2001), 73: 187–198. With 3 figures doi:10.1006/bijl.2001.0536, available online at http://www.idealibrary.com on A molecular phylogeny of the peacock-pheasants (Galliformes: Polyplectron spp.) indicates loss and reduction of ornamental traits and display behaviours REBECCA T. KIMBALL1,2∗, EDWARD L. BRAUN1,3, J. DAVID LIGON1, VITTORIO LUCCHINI4 and ETTORE RANDI4 1Department of Biology, University of New Mexico, Albuquerque, NM 87131, USA 2Department of Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, OH 43210, USA 3Department of Plant Biology, Ohio State University, Columbus, OH 43210, USA 4Istituto Nazionale per la Fauna Selvatica, via Ca` Fornacetta 9, 40064 Ozzano dell’Emilia (BO), Italy Received 4 September 2000; accepted for publication 3 March 2001 The South-east Asian pheasant genus Polyplectron is comprised of six or seven species which are characterized by ocelli (ornamental eye-spots) in all but one species, though the sizes and distribution of ocelli vary among species. All Polyplectron species have lateral displays, but species with ocelli also display frontally to females, with feathers held erect and spread to clearly display the ocelli. The two least ornamented Polyplectron species, one of which completely lacks ocelli, have been considered the primitive members of the genus, implying that ocelli are derived. We examined this hypothesis phylogenetically using complete mitochondrial cytochrome b and control region sequences, as well as sequences from intron G in the nuclear ovomucoid gene, and found that the two least ornamented species are in fact the most recently evolved. Thus, the absence and reduction of ocelli and other ornamental traits in Polyplectronare recent losses. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Lambusango Forest Conservation Project Research Proposal 2008
Lambusango Forest Research Project OPERATION WALLACEA Research Report 2011 Compiled by Dr Philip Wheeler (email [email protected]) University of Hull Scarborough Campus, Centre for Environmental and Marine Sciences Contents Contents .................................................................................................................................................. 1 Introduction ............................................................................................................................................ 2 Research Sites ......................................................................................................................................... 3 Research activity 2011 ............................................................................................................................ 3 Monitoring bird communities............................................................................................................. 4 Bat community dynamics ................................................................................................................... 9 Monitoring herpetofauna and small mammal communities ........................................................... 11 Butterfly community dynamics ........................................................................................................ 15 Monitoring anoa and wild pig populations ...................................................................................... 19 Habitat associations and sleeping site characteristics -

(2004): Identification, Distribution, and Function of Gastroliths in Dinosaurs
IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Diplodocid sauropod accidentally ingesting gastroliths while feeding on a cycad. IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1. Referent: Privat-Dozent Dr. Martin Sander 2. Referent: Professor Dr. Jes Rust Tag der Promotion: 02.12.2004 Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni- bonn.de/diss_online elektronisch publiziert This dissertation is published electronically on the ULB Bonn server for university publications: http://hss.ulb.uni-bonn.de/diss_online Dedicated to Claudia. Thanks for everything. French proverb: “Il a un estomac d’autuche!” (literally: He has the stomach of an ostrich!) means: He can tolerate everything! Geheimnisvoll am lichten Tag Läßt sich Natur des Schleiers nicht berauben, Und was sie deinem Geist