The Fringing Reefs of Magnetic Island: Benthic Biota and Sedimentation - a Baseline Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Coastal Hazard Adaption Strategy for Townsville
Scope and limitations This report: has been prepared by GHD for Townsville City Council and may only be used and relied on by Townsville City Council for the purpose agreed between GHD and the Townsville City Council as set out in section in the project scope of works. GHD otherwise disclaims responsibility to any person other than Townsville City Council arising in connection with this report. GHD also excludes implied warranties and conditions, to the extent legally permissible. The services undertaken by GHD in connection with preparing this report were limited to those specifically detailed in the report and are subject to the scope limitations set out in the report. The opinions, conclusions and any recommendations in this report are based on conditions encountered and information reviewed at the date of preparation of the report. GHD has no responsibility or obligation to update this report to account for events or changes occurring subsequent to the date that the report was prepared. The opinions, conclusions and any recommendations in this report are based on assumptions made by GHD described in this report. GHD disclaims liability arising from any of the assumptions being incorrect. Cover images sourced from Townsville City Council, the Queensland Department of Environment and Heritage Protection and B.Harper (2012). GHD | Report for Townsville City Council - Coastal Hazard Adaptation Strategy, 41/24609 | i Executive summary This study considers the potential ongoing cumulative impacts of coastal hazards on the Townsville regional community in Far North Queensland. It considers both present extremes of climate and also projected changes in future climates up until the year 2100. -

Magnetic Island Townsville &
Cairns Townsville Airlie Beach Rockhampton Brisbane Townsville & Magnetic Island NORTH QUEENSLAND, AUSTRALIA Cairns Townsville Airlie Beach Charters Towers DRIVE TIMES 4hrs Townsville to Cairns Sunshine Coast 3hrs Townsville to Airlie Beach Brisbane 1.5hrs Townsville to Charters Towers Photo courtesy of @wheres yo yo the hidden secret of North Queensland sealinkqld.com.au WELCOME to North Queensland Townsville, the capital of North Queensland, is a vibrant and bustling city surrounded by iconic Australian landscapes of tropical islands, golden beaches, rainforest, wetlands and the outback. Featuring an international airport and national rail connections, it has never been easier to experience life in the tropics! Dive the Yongala Wreck, visit Reef HQ The Ville Resort & Casino Aquarium the world’s largest living coral reef aquarium, join a platypus safari in Paluma or just relax along Townsville’s waterfront precinct The Strand. There are experiences to suit everyone! Townsville is the events capital of North Queensland, with an exciting calendar of sporting, lifestyle, music and marine events showcased each year. Classique B&B Quest on Eyre Reef HQ Wallaman Falls 3 NIGHT TASTE OF TOWNSVILLE 2 NIGHT RAINFOREST SELF DRIVE INCLUSIONS INCLUSIONS • 3 Nights’ Accommodation, Townsville • 2 Nights’ Accommodation, • Tropic Highlights Package Hidden Valley Rustic Cabin – Return ferry transfers • Platypus & solar tour – All day bus pass on Magnetic Island • Guided Nocturnal Night walk – Entry into Bungalow Bay Koala Village, • Hot Breakfast daily Magnetic Island • Self-guided maps of the – Entry into Reef HQ and Museum of Hidden Valley/Paluma area Tropical Queensland in Townsville Hidden Valley Cabins • Townsville Military & Scenic Tour DESCRIPTION DESCRIPTION This self-drive escape is perfect for those seeking Discover the beauty, culture and history of one of the to retreat to a natural setting. -

Scleractinian Reef Corals: Identification Notes
SCLERACTINIAN REEF CORALS: IDENTIFICATION NOTES By JACKIE WOLSTENHOLME James Cook University AUGUST 2004 DOI: 10.13140/RG.2.2.24656.51205 http://dx.doi.org/10.13140/RG.2.2.24656.51205 Scleractinian Reef Corals: Identification Notes by Jackie Wolstenholme is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................................................................ i INTRODUCTION .................................................................................................................................................. 1 ABBREVIATIONS AND DEFINITIONS ............................................................................................................. 2 FAMILY ACROPORIDAE.................................................................................................................................... 3 Montipora ........................................................................................................................................................... 3 Massive/thick plates/encrusting & tuberculae/papillae ................................................................................... 3 Montipora monasteriata .............................................................................................................................. 3 Massive/thick plates/encrusting & papillae ................................................................................................... -

Sexual Reproduction of the Solitary Sunset Cup Coral Leptopsammia Pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean
Marine Biology (2005) 147: 485–495 DOI 10.1007/s00227-005-1567-z RESEARCH ARTICLE S. Goffredo Æ J. Radetic´Æ V. Airi Æ F. Zaccanti Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia: Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis Received: 16 July 2004 / Accepted: 18 December 2004 / Published online: 3 March 2005 Ó Springer-Verlag 2005 Abstract Information on the reproduction in scleractin- came indented, assuming a sickle or dome shape. We can ian solitary corals and in those living in temperate zones hypothesize that the nucleus’ migration and change of is notably scant. Leptopsammia pruvoti is a solitary coral shape may have to do with facilitating fertilization and living in the Mediterranean Sea and along Atlantic determining the future embryonic axis. During oogene- coasts from Portugal to southern England. This coral sis, oocyte diameter increased from a minimum of 20 lm lives in shaded habitats, from the surface to 70 m in during the immature stage to a maximum of 680 lm depth, reaching population densities of >17,000 indi- when mature. Embryogenesis took place in the coelen- viduals mÀ2. In this paper, we discuss the morphological teron. We did not see any evidence that even hinted at aspects of sexual reproduction in this species. In a sep- the formation of a blastocoel; embryonic development arate paper, we report the quantitative data on the an- proceeded via stereoblastulae with superficial cleavage. nual reproductive cycle and make an interspecific Gastrulation took place by delamination. Early and late comparison of reproductive traits among Dend- embryos had diameters of 204–724 lm and 290–736 lm, rophylliidae aimed at defining different reproductive respectively. -

Highways Byways
Highways AND Byways THE ORIGIN OF TOWNSVILLE STREET NAMES Compiled by John Mathew Townsville Library Service 1995 Revised edition 2008 Acknowledgements Australian War Memorial John Oxley Library Queensland Archives Lands Department James Cook University Library Family History Library Townsville City Council, Planning and Development Services Front Cover Photograph Queensland 1897. Flinders Street Townsville Local History Collection, Citilibraries Townsville Copyright Townsville Library Service 2008 ISBN 0 9578987 54 Page 2 Introduction How many visitors to our City have seen a street sign bearing their family name and wondered who the street was named after? How many students have come to the Library seeking the origin of their street or suburb name? We at the Townsville Library Service were not always able to find the answers and so the idea for Highways and Byways was born. Mr. John Mathew, local historian, retired Town Planner and long time Library supporter, was pressed into service to carry out the research. Since 1988 he has been steadily following leads, discarding red herrings and confirming how our streets got their names. Some remain a mystery and we would love to hear from anyone who has information to share. Where did your street get its name? Originally streets were named by the Council to honour a public figure. As the City grew, street names were and are proposed by developers, checked for duplication and approved by Department of Planning and Development Services. Many suburbs have a theme. For example the City and North Ward areas celebrate famous explorers. The streets of Hyde Park and part of Gulliver are named after London streets and English cities and counties. -

AC27 Doc. 12.5
Original language: English AC27 Doc. 12.5 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-seventh meeting of the Animals Committee Veracruz (Mexico), 28 April – 3 May 2014 Interpretation and implementation of the Convention Review of Significant Trade in specimens of Appendix-II species [Resolution Conf. 12.8 (Rev. CoP13)] SELECTION OF SPECIES FOR TRADE REVIEWS FOLLOWING COP16 1. This document has been prepared by the Secretariat. 2. In Resolution Conf. 12.8 (Rev. CoP13) on Review of Significant Trade in specimens of Appendix-II species, the Conference of the Parties: DIRECTS the Animals and Plants Committees, in cooperation with the Secretariat and experts, and in consultation with range States, to review the biological, trade and other relevant information on Appendix-II species subject to significant levels of trade, to identify problems and solutions concerning the implementation of Article IV, paragraphs 2 (a), 3 and 6 (a)... 3. In accordance with paragraph a) of that Resolution under the section Regarding conduct of the Review of Significant Trade, the Secretariat requested UNEP-WCMC to produce a summary from the CITES Trade Database of annual report statistics showing the recorded net level of exports for Appendix-II species over the five most recent years. Its report is attached as Annex 1 (English only) to the present document. The raw data used to prepare this summary are available in document AC27 Inf. 2. 4. Paragraph b) of the same section directs the Animals Committee, on the basis of recorded trade levels and information available to it, the Secretariat, Parties or other relevant experts, to select species of priority concern for review (whether or not such species have been the subject of a previous review). -

Submission Re Proposed Cooloola World Heritage Area Boundary
Nearshore Marine Biodiversity of the Sunshine Coast, South-East Queensland: Inventory of molluscs, corals and fishes July 2010 Photo courtesy Ian Banks Baseline Survey Report to the Noosa Integrated Catchment Association, September 2010 Lyndon DeVantier, David Williamson and Richard Willan Executive Summary Nearshore reef-associated fauna were surveyed at 14 sites at seven locations on the Sunshine Coast in July 2010. The sites were located offshore from Noosa in the north to Caloundra in the south. The species composition and abundance of corals and fishes and ecological condition of the sites were recorded using standard methods of rapid ecological assessment. A comprehensive list of molluscs was compiled from personal observations, the published literature, verifiable unpublished reports, and photographs. Photographic records of other conspicuous macro-fauna, including turtles, sponges, echinoderms and crustaceans, were also made anecdotally. The results of the survey are briefly summarized below. 1. Totals of 105 species of reef-building corals, 222 species of fish and 835 species of molluscs were compiled. Thirty-nine genera of soft corals, sea fans, anemones and corallimorpharians were also recorded. An additional 17 reef- building coral species have been reported from the Sunshine Coast in previous publications and one additional species was identified from a photo collection. 2. Of the 835 mollusc species listed, 710 species could be assigned specific names. Some of those not assigned specific status are new to science, not yet formally described. 3. Almost 10 % (81 species) of the molluscan fauna are considered endemic to the broader bioregion, their known distribution ranges restricted to the temperate/tropical overlap section of the eastern Australian coast (Central Eastern Shelf Transition). -

Vulnerability Assessment and Risk Analysis Full Report
Maunsell Australia Pty Ltd ABN 20 093 846 925 REPORT TOWNSVILLE CITY TOWNSVILLE FLOOD COUNCIL HAZARD ASSESSMENT STUDY Phase 3 Report Vulnerability Assessment and Risk Analysis December 2005 Job No. 80301202.01 AN AECOM COMPANY Townsville Flood Hazard Assessment Study Phase 3 Report Vulnerability Assessment and Risk Analysis Revision Revision Date Details Authorised Name/Position Signature A 08/03/06 Final Issue Brian Wright Original Manager signed by Environment NQ Brian Wright © Maunsell Australia Proprietary Limited 2002 The information contained in this document produced by Maunsell Australia Pty Ltd is solely for the use of the Client identified on the cover sheet for the purpose for which it has been prepared and Maunsell Australia Pty Ltd undertakes no duty to or accepts any responsibility to any third party who may rely upon this document. All rights reserved. No section or element of this document may be removed from this document, reproduced, electronically stored or transmitted in any form without the written permission of Maunsell Australia Pty Ltd. Townsville Flood Hazard Assessment Study Revision A Phase 3 Report – Vulnerability Assessment and Risk Analysis December 2005 J:MMPL\80301202.02\Administration\phase 3\revised final\report.doc Page 2 of 97 Table of Contents Executive Summary 6 1 Introduction 14 1.1 Study Area 15 1.2 Scope of the Study 16 1.3 Acknowledgments 17 2 Establishing the Context 18 2.1 Summary of Project Plan 19 2.1.1 Study Aims and Scope 19 2.2 The Study Structure 20 2.2.1 Risk Management Team 20 2.2.2 Physical -
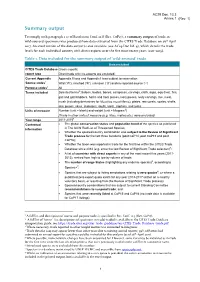
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Scleractinian Corals of the Montebello Islands
Records of the Western Australian Museum Supplement No. 59: 15-19 (2000). SCLERACTINIAN CORALS OF THE MONTEBELLO ISLANDS L.M. Marsh Western Australian Museum, Frands Street, Perth, Western Australia 6000, Australia Summary Montebellos and a further nine from previous The present report lists the first extensive collection records, giving a total of 150 species identified of corals from the Montebello Islands; it is likely that (Table 4). Several species of Montipora, Acropora, many species remain to be found. A total of 141 Favia and Favites remain unidentified and the coral species of 54 genera are recorded from the present fauna is probably still far from completely known, survey and a further 9 species are added from perhaps another 20-30% remains to be discovered. previous records. The coral fauna includes a suite of Seven species of ahermatypic (non zooxanthellate) five genera characteristic of turbid inshore waters but corals were also collected. most of the corals are characteristic of moderately It is probable that many more species will be clear water conditions. The coral fauna overall is found at the Montebellos when the reefs are more most similar to that of the Dampier Archipelago. completely surveyed as many uncommon species may not have been encountered. The species richness of Acropora and Montipora is likely to be Introduction under-recorded because of their great diversity, The first published record of a coral from the polymorphism and taxonomic difficulty. Montebello Islands is by Totton (1952) who figured A suite of species characteristic of upper reef a specimen of Moseleya latistellata collected by Mr fronts exposed to strong wave action (Pocillopora T.H. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of