FAO Species Catalogue. Vol. 4. Sharks of the World. an Annotated And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fishing and Illegal Trade of the Angelshark DNA Barcoding
Fisheries Research 206 (2018) 193–197 Contents lists available at ScienceDirect Fisheries Research journal homepage: www.elsevier.com/locate/fishres The fishing and illegal trade of the angelshark: DNA barcoding against T misleading identifications ⁎ Ingrid Vasconcellos Bunholia, Bruno Lopes da Silva Ferrettea,b, , Juliana Beltramin De Biasia,b, Carolina de Oliveira Magalhãesa,b, Matheus Marcos Rotundoc, Claudio Oliveirab, Fausto Forestib, Fernando Fernandes Mendonçaa a Laboratório de Genética Pesqueira e Conservação (GenPesC), Instituto do Mar (IMar), Universidade Federal de São Paulo (UNIFESP), Santos, SP, 11070-102, Brazil b Laboratório de Biologia e Genética de Peixes (LBGP), Instituto de Biociências de Botucatu (IBB), Universidade Estadual Paulista (UNESP), Botucatu, SP, 18618-689, Brazil c Acervo Zoológico da Universidade Santa Cecília (AZUSC), Universidade Santa Cecília (Unisanta), Santos, SP, 11045-907, Brazil ARTICLE INFO ABSTRACT Handled by J Viñas Morphological identification in the field can be extremely difficult considering fragmentation of species for trade Keywords: or high similarity between congeneric species. In this context, the shark group belonging to the genus Squatina is Conservation composed of three species distributed in the southern part of the western Atlantic. These three species are Endangered species classified in the IUCN Red List as endangered, and they are currently protected under Brazilian law, which Fishing monitoring prohibits fishing and trade. Molecular genetic tools are now used for practical taxonomic identification, parti- Forensic genetics cularly in cases where morphological observation is prevented, e.g., during fish processing. Consequently, DNA fi Mislabeling identi cation barcoding was used in the present study to track potential crimes against the landing and trade of endangered species along the São Paulo coastline, in particular Squatina guggenheim (n = 75) and S. -

Twenty Thousand Parasites Under The
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Departament de Biologia Animal, Biologia Vegetal i Ecologia Tesis Doctoral Twenty thousand parasites under the sea: a multidisciplinary approach to parasite communities of deep-dwelling fishes from the slopes of the Balearic Sea (NW Mediterranean) Tesis doctoral presentada por Sara Maria Dallarés Villar para optar al título de Doctora en Acuicultura bajo la dirección de la Dra. Maite Carrassón López de Letona, del Dr. Francesc Padrós Bover y de la Dra. Montserrat Solé Rovira. La presente tesis se ha inscrito en el programa de doctorado en Acuicultura, con mención de calidad, de la Universitat Autònoma de Barcelona. Los directores Maite Carrassón Francesc Padrós Montserrat Solé López de Letona Bover Rovira Universitat Autònoma de Universitat Autònoma de Institut de Ciències Barcelona Barcelona del Mar (CSIC) La tutora La doctoranda Maite Carrassón Sara Maria López de Letona Dallarés Villar Universitat Autònoma de Barcelona Bellaterra, diciembre de 2016 ACKNOWLEDGEMENTS Cuando miro atrás, al comienzo de esta tesis, me doy cuenta de cuán enriquecedora e importante ha sido para mí esta etapa, a todos los niveles. -

Extinction Risk and Conservation of the World's Sharks and Rays
RESEARCH ARTICLE elife.elifesciences.org Extinction risk and conservation of the world’s sharks and rays Nicholas K Dulvy1,2*, Sarah L Fowler3, John A Musick4, Rachel D Cavanagh5, Peter M Kyne6, Lucy R Harrison1,2, John K Carlson7, Lindsay NK Davidson1,2, Sonja V Fordham8, Malcolm P Francis9, Caroline M Pollock10, Colin A Simpfendorfer11,12, George H Burgess13, Kent E Carpenter14,15, Leonard JV Compagno16, David A Ebert17, Claudine Gibson3, Michelle R Heupel18, Suzanne R Livingstone19, Jonnell C Sanciangco14,15, John D Stevens20, Sarah Valenti3, William T White20 1IUCN Species Survival Commission Shark Specialist Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 2Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 3IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, Newbury, United Kingdom; 4Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, United States; 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom; 6Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Australia; 7Southeast Fisheries Science Center, NOAA/National Marine Fisheries Service, Panama City, United States; 8Shark Advocates International, The Ocean Foundation, Washington, DC, United States; 9National Institute of Water and Atmospheric Research, Wellington, New Zealand; 10Global Species Programme, International Union for the Conservation -

A Practical Guide to Effective Design and Management of Mpas For
A PRACTICAL GUIDE TO THE REPORT EFFECTIVE DESIGN AND 2019 MANAGEMENT OF MPAs FOR SHARKS AND RAYS This project has been a collaboration between the Centre LEAD AUTHOR: for Sustainable Tropical Fisheries and Aquaculture Cassandra L Rigby, James Cook (CSTFA) at James Cook University, Australia, and WWF. University ABOUT WWF AUTHORS: WWF is one of the largest and most experienced Colin Simpendorfer, James Cook independent conservation organizations, with over University 5 million supporters and a global network active in Andy Cornish, WWF-Hong Kong more than 100 countries. WWF´s mission is to stop the degradation of the planet´s natural environment and to build a future in which humans live in harmony with HOW TO CITE THIS WORK: nature, by conserving the world´s biological diversity, Rigby, C.L., Simpfendorfer, C.A. ensuring that the use of renewable resources is and A. Cornish (2019) A Practical sustainable, and promoting the reduction of pollution Guide to Effective Design and and wasteful consumption. WWF works to reverse Management of MPAs for Sharks declining shark populations through Sharks: Restoring and Rays. WWF, Gland, Switzerland. the Balance, a global initiative. www.panda.org DESIGN AND PRODUCTION: sharks.panda.org Evan Jeffries, Catherine Perry – Swim2Birds Ltd ABOUT CSTFA www.swim2birds.co.uk Research within the Centre for Sustainable Tropical Fisheries and Aquaculture (CSTFA) focuses not only Published in May 2019 by WWF on the aquatic and aquaculture systems that produce – World Wide Fund for Nature, food, but also the industries and communities that Gland, Switzerland utilise them. Multidisciplinary collaborations between our researchers provide the synergies to address Any reproduction in full or part substantial research problems in a way that individual must mention the title and credit research groups cannot. -

An Introduction to the Classification of Elasmobranchs
An introduction to the classification of elasmobranchs 17 Rekha J. Nair and P.U Zacharia Central Marine Fisheries Research Institute, Kochi-682 018 Introduction eyed, stomachless, deep-sea creatures that possess an upper jaw which is fused to its cranium (unlike in sharks). The term Elasmobranchs or chondrichthyans refers to the The great majority of the commercially important species of group of marine organisms with a skeleton made of cartilage. chondrichthyans are elasmobranchs. The latter are named They include sharks, skates, rays and chimaeras. These for their plated gills which communicate to the exterior by organisms are characterised by and differ from their sister 5–7 openings. In total, there are about 869+ extant species group of bony fishes in the characteristics like cartilaginous of elasmobranchs, with about 400+ of those being sharks skeleton, absence of swim bladders and presence of five and the rest skates and rays. Taxonomy is also perhaps to seven pairs of naked gill slits that are not covered by an infamously known for its constant, yet essential, revisions operculum. The chondrichthyans which are placed in Class of the relationships and identity of different organisms. Elasmobranchii are grouped into two main subdivisions Classification of elasmobranchs certainly does not evade this Holocephalii (Chimaeras or ratfishes and elephant fishes) process, and species are sometimes lumped in with other with three families and approximately 37 species inhabiting species, or renamed, or assigned to different families and deep cool waters; and the Elasmobranchii, which is a large, other taxonomic groupings. It is certain, however, that such diverse group (sharks, skates and rays) with representatives revisions will clarify our view of the taxonomy and phylogeny in all types of environments, from fresh waters to the bottom (evolutionary relationships) of elasmobranchs, leading to a of marine trenches and from polar regions to warm tropical better understanding of how these creatures evolved. -

1 an Annotated Checklist of the Chondrichthyans of South Africa 1 2 3
1 An annotated checklist of the chondrichthyans of South Africa 2 3 4 DAVID A. EBERT1, 2, 3, 6, SABINE P. WINTNER4 & PETER M. KYNE5 5 6 1 Pacific Shark Research Center, Moss Landing Marine Laboratories, Moss Landing, 7 USA 8 2 South African Institute for Aquatic Biodiversity, Grahamstown, South Africa 9 3 Department of Ichthyology, California Academy of Sciences, San Francisco, USA 10 4 University of KwaZulu-Natal, School of Life Sciences, Durban, South Africa 11 5 Research Institute for the Environment and Livelihoods, Charles Darwin University, 12 Darwin, Australia 13 14 6 Corresponding author: E-mail: [email protected] 15 16 David A. Ebert ORCID ID 0000-0003-4604-8192 17 Sabine P. Wintner ORCID ID 0000-0001-7350-5999 18 Peter M. Kyne ORCID ID 0000-0003-4494-2625 19 20 21 1 1 Abstract 2 3 An annotated checklist of chondrichthyan fishes (sharks, batoids, and chimaeras) 4 occurring in South African waters is presented. The checklist is the result of decades of 5 research and on-going systematic revisions of the regional fauna. The chondrichthyan 6 fauna of South Africa is one of the richest in the world with 191 species, comprising 50 7 families and 103 genera. It consists of 30 families, 64 genera, and 111 species of sharks; 8 17 families, 36 genera, and 72 species of batoids; and, 3 families, 5 genera, and 8 species 9 of chimaeras. The most species-rich shark families are the whaler sharks Carcharhinidae 10 with 20 species followed by the deepwater catsharks Pentanchidae with 13 species. -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Florida's Fintastic Sharks and Rays Lesson and Activity Packet
Florida's Fintastic Sharks and Rays An at-home lesson for grades 3-5 Produced by: This educational workbook was produced through the support of the Indian River Lagoon National Estuary Program. 1 What are sharks and rays? Believe it or not, they’re a type of fish! When you think “fish,” you probably picture a trout or tuna, but fishes come in all shapes and sizes. All fishes share the following key characteristics that classify them into this group: Fishes have the simplest of vertebrate hearts with only two chambers- one atrium and one ventricle. The spine in a fish runs down the middle of its back just like ours, making fish vertebrates. All fishes have skeletons, but not all fish skeletons are made out of bones. Some fishes have skeletons made out of cartilage, just like your nose and ears. Fishes are cold-blooded. Cold-blooded animals use their environment to warm up or cool down. Fins help fish swim. Fins come in pairs, like pectoral and pelvic fins or are singular, like caudal or anal fins. Later in this packet, we will look at the different types of fins that fishes have and some of the unique ways they are used. 2 Placoid Ctenoid Ganoid Cycloid Hard protective scales cover the skin of many fish species. Scales can act as “fingerprints” to help identify some fish species. There are several different scale types found in bony fishes, including cycloid (round), ganoid (rectangular or diamond), and ctenoid (scalloped). Cartilaginous fishes have dermal denticles (Placoid) that resemble tiny teeth on their skin. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -
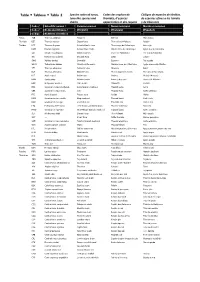
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp