Project 1-1 Project Title: T Cell Immunity in COVID19 Infection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Old Road Campus
Old Road Campus 4a, 4b, 4c, U5 n o t Oxford City Centre g OLD ROAD n i d 4a, 4b, 4c, U5 a e H K O L L A D R O W AD E M I L 4,4a,4b,4c, U1X,U5 A41 42 4,4a,4b,4c,U5 Rin D 4 g R oad 6 7 1 13 3 11 C H U R C H I L L 900 D 2 R I 700, 900 V E E O x f o 5 A C rd 12 C i ty 10 C e n t re B 8 CAR PARK C 9 h u r c R F h i O l l O S H E o V s N E p L i T t DRI a VE ENTRANCE ROOSEVELT DRIVE l 900, ST2 Index 1 The Triangle Nursery 9 Old Road Campus Estates Annexe 13 Boundary Brook House Interserve Joint Research Office Kennedy Institute 2 - Research Services, Medical Sciences Division Old Road Campus Research Building 10 - Clinical Trials and Research Governance 3 New Richards Building Department of Oncology - Human Tissue Governance CRUK/MRC Oxford Institute for Radiation Oncology - Medical Sciences Division Business Development 4 NDM Research Building Institute of Biomedical Engineering Nuffield Department of Primary Care Health Sciences Target Discovery Institute Jenner Institute Medical Sciences Divisional Safety Officers Centre for Tropical Medicine and Global Health Bodleian Knowledge Centre (Library Services) Medical Sciences Division IT Services 5 Wellcome Centre for Human Genetics (WHG) Ludwig Institute for Cancer Research Structural Genomics Consortium 6 Henry Wellcome Building for Molecular Physiology Nuffield Department of Surgical Sciences Loading Bays and Delivery Offices of the Nuffield Professor of Medicine ENTRANCE VIA BUILDING 5 11 Big Data Institute A Wellcome Trust Centre for Human Genetics 7 Henry Wellcome Building for Particle Imaging -

The 6-In-1 Vaccine Study
Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk The 6-in-1 vaccine study Study Information Booklet We are inviting infants aged 8-13 weeks of age to take part in a study comparing two licensed combination vaccines that can be given in the routine UK immunisation schedule. These vaccines protect against 6 different infections in the one injection. Before you decide to take part in this study, it is important for you to understand what the study is about and what participation would involve. Please take time to read the information carefully, and discuss with others if you wish. If you have any questions please contact the study team. Thank you for taking the time to consider this study. Contact the local study team at: Oxford Vaccine Group CCVTM, Churchill Hospital, Oxford, OX3 7LE 01865 611400 [email protected] www.ovg.ox.ac.uk/recruiting-studies Page 1 of 11 The 6-in-1 vaccine study; OVG 2018/05; Participant Information Booklet; REC Ref: 19/SC/0052; IRAS ID: 252324; Version 2.0 dated 23-APR-2019 Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk Summary • This study will help us to better understand the interaction between ‘6- in-1’ vaccines and the Meningococcal B vaccine in the routine UK immunisation schedule • We will be enrolling 240 healthy babies aged 8 – 13 weeks into this study • Babies will receive all their infant immunisations in their own home or in a convenient location until 12 months of age • There will be six study visits including 4 vaccine visits and 2 visits for blood sampling (we will use a topical anaesthetic cream to numb the skin for blood sampling) Why has my child been invited to take part? You have been approached as your child is in the age range for this study and lives in the Thames Valley. -

CURRICULUM VITAE Floyd L
CURRICULUM VITAE Floyd L. Wormley Jr., Ph.D. Office of Research and Graduate Studies Texas Christian University TCU Box 297024 Fort Worth, TX 76129 Phone: (817) 257-7104 ADDRESS: 7121 Axis Ct. Fort Worth, TX 76132 Tel: (817) 386-0645, E-mail: [email protected] ACADEMIC TRAINING: 2002-2005 Doctoral Fellowship, Infectious Diseases Duke University Medical Center, Durham, NC Interdisciplinary Research Training Program in AIDS Program in Microbial Pathogenesis of Cryptococcus neoformans Research Project: Study of host-pathogen interactions during Cryptococcus neoformans infection. Fellowship Advisor: John R. Perfect, M.D. 1998-2001 Doctor of Philosophy, Microbiology/Immunology Louisiana State University Health Sciences Center, New Orleans, LA Dissertation Title: Cell Mediated Immunity Against Experimental Candida albicans Vaginitis. Dissertation Advisor: Paul L. Fidel Jr., Ph.D. 1995-1998 Master of Science, Microbiology/Immunology Louisiana State University Health Sciences Center, New Orleans, LA Thesis Title: Evidence for a Unique CD4 Protein on Murine Vaginal CD4+ T Cells. Thesis Advisor: Paul L. Fidel Jr., Ph.D. 1990-1995 Bachelor of Science, Cellular and Molecular Biology Tulane University, New Orleans, LA. EMPLOYMENT: 2019-Present Associate Provost for Research and Dean of Graduate Studies, Texas Christian University, Fort Worth, TX Role and Responsibility – Promote the advancement of research within the institution and administer key compliance programs through which we promote the responsible conduct of research and integrity. I oversee all research policies and procedures, institutional research compliance committees, and research technology and innovation. I also oversee services and programs for those who seek financial support for their scholarly endeavors from inception of idea through submission and award of research/scholarly project. -

Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2
animals Review Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2. Fetomaternal Response to Infection and Novel Diagnostic Perspectives Paulina Jawor 1,* , John F. Mee 2 and Tadeusz Stefaniak 1 1 Department of Immunology, Pathophysiology and Veterinary Preventive Medicine, Wrocław University of Environmental and Life Sciences, 50-375 Wrocław, Poland; [email protected] 2 Animal and Bioscience Research Department, Teagasc, Moorepark Research Centre, P61 P302 Fermoy, County Cork, Ireland; [email protected] * Correspondence: [email protected] Simple Summary: Bovine perinatal mortality (death of the fetus or calf before, during, or within 48 h of calving at full term (≥260 days) may be caused by noninfectious and infectious causes. Although infectious causes of fetal mortality are diagnosed less frequently, infection in utero may also compromise the development of the fetus without causing death. This review presents fetomaternal responses to infection and the changes which can be observed in such cases. Response to infection, especially the concentration of immunoglobulins and some acute-phase proteins, may be used for diagnostic purposes. Some changes in internal organs may also be used as an indicator of infection in utero. However, in all cases (except pathogen-specific antibody response) non-pathogen-specific responses do not aid in pathogen-specific diagnosis of the cause of calf death. But, nonspecific markers of in utero infection may allow us to assign the cause of fetal mortality to infection and thus Citation: Jawor, P.; Mee, J.F.; increase our overall diagnosis rate, particularly in cases of the “unexplained stillbirth”. Stefaniak, T. Role of Infection and Immunity in Bovine Perinatal Abstract: Bovine perinatal mortality due to infection may result either from the direct effects of Mortality: Part 2. -
Astrazeneca-Oxford Vaccine Approved for Use in the U.K
P2JW366000-6-A00100-17FFFF5178F ****** THURSDAY,DECEMBER 31,2020~VOL. CCLXXVI NO.154 WSJ.com HHHH $4.00 DJIA 30409.56 À 73.89 0.2% NASDAQ 12870.00 À 0.2% STOXX 600 400.25 g 0.3% 10-YR. TREAS. À 3/32 , yield 0.926% OIL $48.40 À $0.40 GOLD $1,891.00 À $10.50 EURO $1.2300 YEN 103.21 Deadly Attack at Airport Targets New Yemen Government U.S. IPO What’s News Market Reaches Business&Finance Record nvestorspiled into IPOs Iat a record rate in 2020, with companies raising Total $167.2 billion via 454 of- ferings on U.S. exchanges this year through Dec. 24. Few see signs of letup Few expect the euphoria after companies raise to wear off soon. A1 more than $167 billion Detenteisending in the global fight over tech taxes, despite pandemic with Franceresuming collec- tion of itsdigital-services tax BY MAUREEN FARRELL and the U.S. poised to retali- atewith tariffs.Other coun- Defying expectations,inves- tries areset to join the fray. A1 S tors piled intoinitial public of- China finished 2020 PRES feringsatarecordrateiN with a 10th consecutive TED 2020, and few expect the eu- month of expansion in its CIA phoria to wear off soon. manufacturing sector. A7 SO Companies raised $167.2 AS TheEUand China agreed TENSIONS HIGH: People fled after an explosion Wednesday at the airport in Aden, Yemen, moments after members of the billion through 454 offerings in principle on an invest- country’s newly sworn-in cabinet arrived. At least 22 people were killed, but all the members of the cabinet were safe. -

La Verdad De La Pandemia Os Gusta Y Seguís Siendo Mis Mecenas, Pronto Nos Pondremos a Trabajar En El Siguiente Libro
Índice Portada Sinopsis Dedicatoria Cita INTRODUCCIÓN. EL PRINCIPIO DEL CAOS PRIMERA PARTE. HECHOS 1. «TODO ESTÁ BAJO CONTROL» The Economist dixit Wuhan: un suceso local de alcance global El banco de virus más grande de Asia Cifras y muertes que no cuadran… La «orden»: confinamiento de la población El confinamiento de Wuhan… … Y China tiene el control El papel de la OMS Un espejo para comprender: Taiwán 2. HISTORIA DE UNA INFAMIA La OMS se alinea con el poder El «profeta»Tedros Adhanom La ideología del poder: el «Informe Kissinger» y los globócratas Nos matan con vacunas: la infertilidad de las mujeres Gursaran Pran Talwar y la vacuna anticonceptiva Bill Gates, vacunas y demografía La pandemia de Bill Gates La vacuna de Gates para el coronavirus SEGUNDA PARTE. LA IDEOLOGÍA DE LA ÉLITE 3. LABORATORIOS DE MANIPULACIÓN SOCIAL La Escuela de Chicago Mass Communication Research La Escuela de Fráncfort Tavistock Institute Estudios Culturales Método crítico versus colaboración con la superélite El MIT: el laboratorio actual Alex Pentland, el gurú de la élite globalista El MIT como faro de la élite Los amos del mundo iniciaron el camino: el Club Bilderberg 4. MEDIOS DE COMUNICACIÓN Y MENTIRAS Poder y comunicación: algunos apuntes históricos La Antigüedad La Edad Media La Edad Moderna La Edad Contemporánea Comunicación y cultura de masas Manipulación y propaganda El poder del cine La globalización: ¿quién tiene el poder? La «sociedad red» La comunicación en la «sociedad red» Normópatas El poder mediático y sus tentáculos La ideología de los medios de comunicación y la creación de alianzas Alphabet (Google), Amazon, Facebook, Apple, Microsoft y Netflix La sociedad domesticada con el mensaje único Los grandes conglomerados mediáticos y el Club Bilderberg Jeff Bezos, The Washington Post y Amazon Eric Schmidt, YouTube y Google Facebook, mucho más que una red social Mark Zuckerberg y Bilderberg: los dueños de Facebook Los jueces TERCERA PARTE. -

Annual Meeting
Volume 97 | Number 5 Volume VOLUME 97 NOVEMBER 2017 NUMBER 5 SUPPLEMENT SIXTY-SIXTH ANNUAL MEETING November 5–9, 2017 The Baltimore Convention Center | Baltimore, Maryland USA The American Journal of Tropical Medicine and Hygiene The American Journal of Tropical astmh.org ajtmh.org #TropMed17 Supplement to The American Journal of Tropical Medicine and Hygiene ASTMH FP Cover 17.indd 1-3 10/11/17 1:48 PM Welcome to TropMed17, our yearly assembly for stimulating research, clinical advances, special lectures, guests and bonus events. Our keynote speaker this year is Dr. Paul Farmer, Co-founder and Chief Strategist of Partners In Health (PIH). In addition, Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, will deliver a plenary session Thursday, November 9. Other highlighted speakers include Dr. Scott O’Neill, who will deliver the Fred L. Soper Lecture; Dr. Claudio F. Lanata, the Vincenzo Marcolongo Memorial Lecture; and Dr. Jane Cardosa, the Commemorative Fund Lecture. We are pleased to announce that this year’s offerings extend beyond communicating top-rated science to direct service to the global community and a number of novel events: • Get a Shot. Give a Shot.® Through Walgreens’ Get a Shot. Give a Shot.® campaign, you can not only receive your free flu shot, but also provide a lifesaving vaccine to a child in need via the UN Foundation’s Shot@Life campaign. • Under the Net. Walk in the shoes of a young girl living in a refugee camp through the virtual reality experience presented by UN Foundation’s Nothing But Nets campaign. -
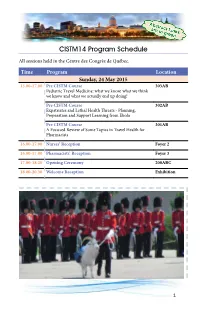
CISTM14 Program Schedule
CISTM14 Program Schedule All sessions held in the Centre des Congrès de Québec. Time Program Location Sunday, 24 May 2015 13.00-17.00 Pre CISTM Course 303AB Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! Pre CISTM Course 302AB Expatriates and Lethal Health Threats - Planning, Preparation and Support Learning from Ebola Pre CISTM Course 301AB A Focused Review of Some Topics in Travel Health for Pharmacists 16.00-17.00 Nurses’ Reception Foyer 2 16.00-17.00 Pharmacists’ Reception Foyer 3 17.00-18.20 Opening Ceremony 200ABC 18.00-20.30 Welcome Reception Exhibition 1 Time Program Location Monday, 25 May 2015 MTH1 Meet The History 301AB 8.00-8.45 History of the Quarantine Station at Grosse Ile Marc Desmeules, Canada 8.00-8.45 CDC Yellow Book 302AB Gary Brunette, United States of America COD1 Case of the Day 303AB 8.00-8.45 Pre-Travel Rogelio Lopez-Velez, Spain PL1 Plenary 200ABC 9.00-10.30 Our Shrinking World: Health in the 21st Century Chairs: David R. Shlim, United States of America Leo Visser, The Netherlands PL1.01 Measuring global health: The global is local [Change your mindset! It’s time for a reality check] Louis Loutan, Switzerland • Outline the most important trends and health challenges in the world using gapminder/health metrics • Appraise how these changes (global health transitions, global population growth, urbanization, changes in animal helath and human-animal population interactions, and climate change) will influence global migration and travel medicine now and in the future • Integrate global health concepts into thinking about travel medicine 2 Time Program Location Monday, 25 May 2015, continued Cont. -

Sir Bryn Terfel Premieres John Rutter's 'Joseph's Carol', Dedicated to the Oxford Vaccine Team in Celebratory Concert Fr
Sir Bryn Terfel premieres John Rutter’s ‘Joseph’s Carol’, dedicated to the Oxford vaccine team in celebratory concert from the Oxford Philharmonic Orchestra Friday 18 December 2020, 18:30 Streamed on Oxford Philharmonic Orchestra’s YouTube Channel: bit.ly/OPOVaccineTribute Elgar Chanson de Matin William Henry Monk Abide with Me Rodgers & Hammerstein You’ll Never Walk Alone John Rutter Joseph’s Carol WORLD PREMIERE John Rutter Look to the Day Handel Hallelujah Chorus Sir Bryn Terfel bass-baritone Oxford Philharmonic Orchestra Maxim Vengerov violin Choir of Merton College, Oxford John Rutter conductor Alexandra Lowe soprano Marios Papadopoulos conductor Alexander Olleson treble John Suchet presenter In recognition of the formidable work accomplished by the team of scientists at the University of Oxford on their Covid-19 vaccine, the Oxford Philharmonic Orchestra will stream a celebratory concert on Friday 18 December, recorded in the city’s historic Sheldonian Theatre. Performed by bass-baritone Sir Bryn Terfel, the short concert features the premiere of John Rutter’s Joseph’s Carol, written in tribute to the Oxford Vaccine Group, the Jenner Institute and the RECOVERY team. The words by John Rutter recount the long and weary journey of Joseph and Mary to Bethlehem before the birth of the baby Jesus, echoing the programme’s journey from struggle through to hope. Bryn Terfel also joins the Orchestra and the Choir of Merton College, Oxford, in a rousing programme from Rodgers & Hammerstein’s You’ll Never Walk Alone (with Jette Parker Young Artist Alexandra Lowe) to Handel’s Hallelujah Chorus. Sir Bryn and the Orchestra are also joined in the hymn of comfort, Abide with Me, by chorister Alexander Olleson of Christ Church Cathedral Choir, the recent winner of BBC Young Chorister Of The Year 2020. -

Mbio-2016-Casadevall-.Pdf
Downloaded from EDITORIAL Rigorous Science: a How-To Guide mbio.asm.org Arturo Casadevall,a Founding Editor in Chief, mBio, Ferric C. Fang,b Editor in Chief, Infection and Immunity Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USAa; Departments of Laboratory on January 3, 2017 - Published by Medicine and Microbiology, University of Washington School of Medicine, Seattle, Washington, USAb ABSTRACT Proposals to improve the reproducibility of biomedical research have emphasized scientific rigor. Although the word “rigor” is widely used, there has been little specific discussion as to what it means and how it can be achieved. We suggest that scientific rigor combines elements of mathematics, logic, philosophy, and ethics. We propose a framework for rigor that includes redundant experimental design, sound statistical analysis, recognition of error, avoidance of logical fallacies, and intel- lectual honesty. These elements lead to five actionable recommendations for research education. igor is a prized quality in scientific work. Although the term is ciple, such as Schrödinger’s cat or Maxwell’s demon in physics, or Rwidely used in both scientific and lay parlance, it has not been entirely experimental, as illustrated by Cavendish’s measurement precisely defined (1). Rigor has gained new prominence amid con- of the gravitational constant at the end of the 18th century. How- mbio.asm.org cerns about a lack of reproducibility in important studies (2, 3), an ever, in the biomedical sciences, most research has both theoreti- epidemic of retractions due to misconduct (4), and the discovery cal and experimental aspects. that the published literature is riddled with problematic images (5). -

The 100 Top-Cited Tuberculosis Research Studies
INT J TUBERC LUNG DIS 19(6):717–722 Q 2015 The Union http://dx.doi.org/10.5588/ijtld.14.0925 The 100 top-cited tuberculosis research studies L.-M. Chen,*† Y.-Q. Liu,* J.-N. Shen,* Y.-L. Peng,* T.-Y. Xiong,* X. Tong,‡ L. Du,*§¶ Y.-G. Zhang*§¶ *West China Hospital, West China Medical School, Sichuan University, Chengdu, †Department of Anaesthesiology, West China Hospital of Sichuan University, Chengdu, ‡Department of Respiratory Medicine, West China Hospital of Sichuan University, Chengdu, §The Periodical Press of West China Hospital, Sichuan University, Chengdu, ¶The Chinese Cochrane Centre, West China Hospital, Sichuan University, Chengdu, China SUMMARY The examination of top-cited studies is a useful method ing author. The United States contributed the largest for identify and monitoring outstanding scientific number of studies, followed by the United Kingdom and research. The objective of this study was to identify France. The institutions with the largest number of and analyse the characteristics of the top 100 cited articles were the Institut National de la Santeetdela´ research studies on tuberculosis (TB) based on the Web Recherche Medicale´ in France and the University of of Knowledge. Overall, the top 100 cited studies were California in the United States. The studies appeared in cited between 366 and 4443 times, and were published 35 journals, with 11 published in Science, followed by between 1995 and 2010, with the largest number of PNAS and NEJM. The majority of TB articles have been publications in 2003 and in 1995. Four studies were published in those medical journals with the highest attributed to a single author and 10 to two authors; the impact factors, and are from the most industrialised number of authors exceeded six in 50 studies. -

'Astrazeneca' Covid-19 Vaccine
Medicines Law & Policy How the ‘Oxford’ Covid-19 vaccine became the ‘AstraZeneca’ Covid-19 vaccine By Christopher Garrison 1. Introduction. The ‘Oxford / AstraZeneca’ vaccine is one of the world’s leading hopes in the race to end the Covid-19 pandemic. Its history is not as clear, though, as it may first seem. The media reporting about the vaccine tends to focus either on the very small (non-profit, academic) Jenner Institute at Oxford University, where the vaccine was first invented, or the very large (‘Big Pharma’ firm) AstraZeneca, which is now responsible for organising its (non-profit) world-wide development, manufacture and distribution. However, examining the intellectual property (IP) path of the vaccine from invention to manufacture and distribution reveals a more complex picture that involves other important actors (with for-profit perspectives). Mindful of the very large sums of public money being used to support Covid-19 vaccine development, section 2 of this note will therefore contextualise the respective roles of the Jenner Institute, AstraZeneca and these other actors, so that their share of risk and (potential) reward in the project can be better understood. Section 3 provides comments as well as raising some important questions about what might yet be done better and what lessons can be learned for the future. 2. History of the ‘Oxford / AstraZeneca’ vaccine. 2.1 Oxford University and Oxford University Innovation Ltd. The Bayh-Dole Act (1980) was hugely influential in the United States and elsewhere in encouraging universities to commercially exploit the IP they were generating by setting up ‘technology transfer’ offices.