Graham-Cumming J. the Geek Atlas (O`Reilly, 2009)(ISBN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elsecar Conservation Area Appraisal Workshop Held: 19Th February 2008 at Milton Hall
Elsecar Conservation Area Appraisal Version 1.1 1. Introduction Elsecar • Explanation of a Conservation Area, relevant guidance and why an appraisal has been undertaken 2. Location and Scope of Conservation Area • Landscape and surrounding setting • General character and plan form 3. Historical Context and Development • Origins and historical development of the area • Archaeology 4. Townscape Analysis • Current Uses/ Activities • Prominent buildings/ Landmarks • Open Space • Public Realm • Buildings that have a positive, neutral or negative effect on the character of the Conservation Area • Sites that have a positive, neutral or negative effect on the character of the Conservation Area • The character and contribution of Green spaces and their relative biodiversity value 5. Architectural Analysis • Local building materials • Vernacular styles • Key unlisted buildings/ types or groups of unlisted buildings and features • Listed buildings • Aesthetic Appeal of buildings and spaces • The relationship between buildings within the Conservation Area 6. The Character and Appearance of the Conservation Area • Summary of the current character and appearance of the area 7. Proposals for the Future Preservation and Enhancement of the Conservation Area • Policy background • Specific policy guidance for future development in the Conservation Area • Evaluation of proposals for future extension of the Conservation Area 1 Version 1.1 8. Glossary of Terms Elsecar Further Reading Appendices • Appendix A Listed Building Profiles • Appendix B Ancient Monuments profiles • Appendix C Report on Consultation Undertaken Contact Details 2 Version 1.1 1. Introduction Figure 1: The location of Elsecar in relation to surrounding settlements Elsecar 1.1. This document is an appraisal of the Conservation Area that covers the village of Elsecar around 6 miles to the south east of Barnsley. -

MACHINES OR ENGINES, in GENERAL OR of POSITIVE-DISPLACEMENT TYPE, Eg STEAM ENGINES
F01B MACHINES OR ENGINES, IN GENERAL OR OF POSITIVE-DISPLACEMENT TYPE, e.g. STEAM ENGINES (of rotary-piston or oscillating-piston type F01C; of non-positive-displacement type F01D; internal-combustion aspects of reciprocating-piston engines F02B57/00, F02B59/00; crankshafts, crossheads, connecting-rods F16C; flywheels F16F; gearings for interconverting rotary motion and reciprocating motion in general F16H; pistons, piston rods, cylinders, for engines in general F16J) Definition statement This subclass/group covers: Machines or engines, in general or of positive-displacement type References relevant to classification in this subclass This subclass/group does not cover: Rotary-piston or oscillating-piston F01C type Non-positive-displacement type F01D Informative references Attention is drawn to the following places, which may be of interest for search: Internal combustion engines F02B Internal combustion aspects of F02B 57/00; F02B 59/00 reciprocating piston engines Crankshafts, crossheads, F16C connecting-rods Flywheels F16F Gearings for interconverting rotary F16H motion and reciprocating motion in general Pistons, piston rods, cylinders for F16J engines in general 1 Cyclically operating valves for F01L machines or engines Lubrication of machines or engines in F01M general Steam engine plants F01K Glossary of terms In this subclass/group, the following terms (or expressions) are used with the meaning indicated: In patent documents the following abbreviations are often used: Engine a device for continuously converting fluid energy into mechanical power, Thus, this term includes, for example, steam piston engines or steam turbines, per se, or internal-combustion piston engines, but it excludes single-stroke devices. Machine a device which could equally be an engine and a pump, and not a device which is restricted to an engine or one which is restricted to a pump. -

Building a Small Horizontal Steam Engine
Building a Small Horizontal Steam Engine The front cylinder head is a pipe cap, THE small engine described in this the exterior of which is turned to pre- article was built by the writer in sent a more pleasing appearance, and his spare time—about an hour a day for drilled and threaded to receive the stuff- four months—and drives the machinery ing box, Fig. 2. The distance between in a small shop. At 40-lb. gauge pres- the edge of the front-end steam port and sure, the engine runs at 150 r.p.m., under the inner side of the cap, when screwed full load, and delivers a little over .4 home, should be much less than that brake horsepower. A cast steam chest, shown, not over ¼ in., for efficiency, and with larger and more direct steam ports, the same at the rear end. When the to reduce condensation losses; less clear- cap has been permanently screwed on ance in the cylinder ends, and larger the cylinder, one side is flattened, as bearing surfaces in several places, would shown, on the shaper or grinder, and the bring the efficiency of the engine up to a steam ports laid out and drilled. It would much higher point than this. In the be a decided advantage to make these writer's case, however, the engine is de- ports as much larger than given as is livering ample power for the purpose to possible, as the efficiency with ½-in. ports which it is applied, and consequently is far below what it might be. -

Crypto Review
Crypto Review Track 3: Security Workshop Overview • What is Cryptography? • Symmetric Key Cryptography • Asymmetric Key Cryptography • Block and Stream Cipher • Digital Signature and Message Digest Cryptography • Cryptography is everywhere German Lorenz cipher machine Cryptography • Cryptography deals with creang documents that can be shared secretly over public communicaon channels • Other terms closely associated – Cryptanalysis = code breaking – Cryptology • Kryptos (hidden or secret) and Logos (descripGon) = secret speech / communicaon • combinaon of cryptography and cryptanalysis • Cryptography is a funcGon of plaintext and a Notaon: Plaintext (P) cryptographic key Ciphertext (C) C = F(P, k) Cryptographic Key (k) Typical Scenario • Alice wants to send a “secret” message to Bob • What are the possible problems? – Data can be intercepted • What are the ways to intercept this message? • How to conceal the message? – Encrypon Crypto Core • Secure key establishment Alice has key (k) Bob has key (k) • Secure communicaon m Confidenality and integrity m m Alice has key (k) Bob has key (k) Source: Dan Boneh, Stanford It can do much more • Digital Signatures • Anonymous communicaon • Anonymous digital cash – Spending a digital coin without anyone knowing my idenGty – Buy online anonymously? • ElecGons and private aucGons – Finding the winner without actually knowing individual votes (privacy) Source: Dan Boneh, Stanford Other uses are also theoreGcally possible (Crypto magic) What did she • Privately outsourcing computaon search for? E(query) -

Assessing Steam Locomotive Dynamics and Running Safety by Computer Simulation
TRANSPORT PROBLEMS 2015 PROBLEMY TRANSPORTU Volume 10 Special Edition steam locomotive; balancing; reciprocating; hammer blow; rolling stock and track interaction Dāvis BUŠS Institute of Transportation, Riga Technical University Indriķa iela 8a, Rīga, LV-1004, Latvia Corresponding author. E-mail: [email protected] ASSESSING STEAM LOCOMOTIVE DYNAMICS AND RUNNING SAFETY BY COMPUTER SIMULATION Summary. Steam locomotives are preserved on heritage railways and also occasionally used on mainline heritage trips, but since they are only partially balanced reciprocating piston engines, damage is made to the railway track by dynamic impact, also known as hammer blow. While causing a faster deterioration to the track on heritage railways, the steam locomotive may also cause deterioration to busy mainline tracks or tracks used by high speed trains. This raises the question whether heritage operations on mainline can be done safely and without influencing the operation of the railways. If the details of the dynamic interaction of the steam locomotive's components are examined with computerised calculations they show differences with the previous theories as the smaller components cannot be disregarded in some vibration modes. A particular narrow gauge steam locomotive Gr-319 was analyzed and it was found, that the locomotive exhibits large dynamic forces on the track, much larger than those given by design data, and the safety of the ride is impaired. Large unbalanced vibrations were found, affecting not only the fatigue resistance of the locomotive, but also influencing the crew and passengers in the train consist. Developed model and simulations were used to check several possible parameter variations of the locomotive, but the problems were found to be in the original design such that no serious improvements can be done in the space available for the running gear and therefore the running speed of the locomotive should be limited to reduce its impact upon the track. -

Translation Rights List Non-Fiction May 2019
Translation Rights List Including Non-Fiction May 2019 Contents • Rights Department p.3 • Little, Brown Imprints p.4 • General p.5 • Culture p.13 • Business & Management p.19 • History p.22 • Music p.28 • Memoirs & Biography p.34 • Sport p.39 • Travel & Nature p.41 • Health, Self-Help & Popular Psychology p.42 • Parenting p.48 • Food & Cookery p.53 • Overcoming Series p.56 • Rights Representatives p.59 Key • Rights sold displayed in parentheses indicates that we do not control the rights • An asterisk indicates a new title since previous Rights list • Titles in italics were not published by Little, Brown Book Group 2 Rights Department ANDY HINE Rights Director Brazil, Germany, Italy, Poland, Scandinavia, Latin America and the Baltic States [email protected] +44 (0) 20 3122 6545 KATE HIBBERT Rights Director USA, Spain, Portugal, Far East, the Netherlands, Flemish Belgium and the Indian Subcontinent [email protected] +44 (0) 20 3122 6619 HELENA DOREE Senior Rights Manager France, French Belgium, Turkey, Arab States, Israel, Greece, Bulgaria, Czech Republic, Slovak Republic, Hungary, Romania, Russia, Serbia and Macedonia [email protected] +44 (0) 20 3122 6598 HENA BRYAN Rights Trainee [email protected] +44 (0) 20 3122 0693 Little, Brown Book Group Follow us on Twitter: Carmelite House @LBBGRights 50 Victoria Embankment London EC4Y 0DZ UNITED KINGDOM 3 Little, Brown Imprints 4 General Highlights WHEN THE CLOUDS FELL FROM THE SKY THE ADVENTURES OF AZUKI 5 THE ADVENTURES OF AZUKI THE MINIATURE HEDGEHOG Humour| Robinson | 112pp |60 colour photographs| September 2019 A full-colour collection of photographs of the adorable Azuki, the miniature hedgehog, enjoying some of his favourite adventures, from camping to baking Riding on the popularity of everyone's favourite Insta-famous miniature hedgehog (400,000 followers on Instagram and counting), this adorable illustrated book stands out as a gem of originality among the glut of dog and cat books. -

Support Barnsley's Museums
Cannon Hall is a stunning Georgian country house museum with outstanding fine and decorative art collections, set in Barnsley Museums 70 acres of historic parkland and beautiful landscaped gardens. It is the perfect day out for all the family. is made up of five magnificent Bark House Lane, Cawthorne, Barnsley, S75 4AT [email protected] attractions. All are 01226 772002 • www.cannon-hall.com free to enter and each has its own The Cooper Gallery is a vibrant art gallery in the heart unique identity. of Barnsley town centre. Explore galleries showcasing the beautiful collection of fine art and enjoy our exciting programme of temporary exhibitions, events and arts workshops. Cooper Gallery, Church Street, Barnsley, S70 2AH [email protected] 01226 242905 • www.cooper-gallery.com As well as soaking up the warm, spring as the celebration of one of Barnsley’s sunshine - why not soak up the relaxing influential industries in the exhibition visitors can uncover the incredible Experience Barnsley atmosphere at Barnsley Museums’ five Tins!Tins!Tins! at Experience Barnsley - story of Barnsley told through centuries-old artefacts, fantastic sites. there is something for everyone to enjoy. documents, films and recordings that have been donated by people living and working in the borough. Whether you are hankering after the great So if you are looking for unique outdoors, inspiring local history, a trip out destinations to spend quality time with Town Hall, Church Street, Barnsley, S70 2TA with the family, art exhibitions, fun events, family, friends and loved ones this spring, [email protected] retail therapy or even a bite to eat, then Barnsley’s museums are the perfect place look no further as Barnsley Museums have for you. -

Warwickshire Industrial Archaeology Society
WARWICKSHIRE IndustrialW ArchaeologyI SociASety NUMBER 31 June 2008 PUBLISHED QUARTERLY NEWSLETTER THIS ISSUE it was felt would do nothing to web site, and Internet access further these aims and might becoming more commonplace ¢ Meeting Reports detract from them, as if the amongst the Society membership, current four page layout were what might be the feelings of ¢ From The Editor retained, images would reduce the members be towards stopping the space available for text and practice of posting copies to possibly compromise the meeting those unable to collect them? ¢ Bridges Under Threat reports. Does this represent a conflict This does not mean that with the main stated aim of ¢ Meetings Programme images will never appear in the publishing a Newsletter, namely Newsletter. If all goes to plan, that of making all members feel this edition will be something of a included in the activities of the FROM THE EDITOR milestone since it will be the first Society? y editorial in the to contain an illustration; a Mark Abbott March 2008 edition of diagram appending the report of Mthis Newsletter the May meeting. Hopefully, PROGRAMME concerning possible changes to its similar illustrations will be format brought an unexpected possible in future editions, where Programme. number of offers of practical appropriate and available, as the The programme through to help. These included the offer of technology required to reproduce December 2008 is as follows: a second hand A3 laser printer at them is now quite September 11th a very attractive price; so straightforward. The inclusion of Mr. Lawrence Ince: attractive as to be almost too photographs is not entirely ruled Engine-Building at Boulton and good an opportunity to ignore. -
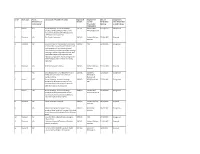
Serial Asset Type Active Designation Or Undertaking?
Serial Asset Type Active Description of Record or Artefact Registered Disposal to / Date of Designation, Designation or Number Current Designation Class Designation Undertaking? Responsible Meeting or Undertaking Organisation 1 Record YES Brunel Drawings: structural drawings 1995/01 Network Rail 22/09/1995 Designation produced for Great Western Rly Co or its Infrastructure Ltd associated Companies between 1833 and 1859 [operational property] 2 Disposed NO The Gooch Centrepiece 1995/02 National Railway 22/09/1995 Disposal Museum 3 Replaced NO Classes of Record: Memorandum and Articles 1995/03 N/A 24/11/1995 Designation of Association; Annual Reports; Minutes and working papers of main board; principal subsidiaries and any sub-committees whether standing or ad hoc; Organisation charts; Staff newsletters/papers and magazines; Files relating to preparation of principal legislation where company was in lead in introducing legislation 4 Disposed NO Railtrack Group PLC Archive 1995/03 National Railway 24/11/1995 Disposal Museum 5 YES Class 08 Locomotive no. 08616 (formerly D 1996/01 London & 22/03/1996 Designation 3783) (last locomotive to be rebuilt at Birmingham Swindon Works) Railway Ltd 6 Record YES Brunel Drawings: structural drawings 1996/02 BRB (Residuary) 22/03/1996 Designation produced for Great Western Rly Co or its Ltd associated Companies between 1833 and 1859 [Non-operational property] 7 Record YES Brunel Drawings: structural drawings 1996/02 Network Rail 22/03/1996 Designation produced for Great Western Rly Co or its Infrastructure -

Physics 305, Fall 2008 Problem Set 8 Due Thursday, December 3
Physics 305, Fall 2008 Problem Set 8 due Thursday, December 3 1. Einstein A and B coefficients (25 pts): This problem is to make sure that you have read and understood Griffiths 9.3.1. Consider a system that consists of atoms with two energy levels E1 and E2 and a thermal gas of photons. There are N1 atoms with energy E1, N2 atoms with energy E2 and the energy density of photons with frequency ! = (E2 − E1)=~ is W (!). In thermal equilbrium at temperature T , W is given by the Planck distribution: ~!3 1 W (!) = 2 3 : π c exp(~!=kBT ) − 1 According to Einstein, this formula can be understood by assuming the following rules for the interaction between the atoms and the photons • Atoms with energy E1 can absorb a photon and make a transition to the excited state with energy E2; the probability per unit time for this transition to take place is proportional to W (!), and therefore given by Pabs = B12W (!) for some constant B12. • Atoms with energy E2 can make a transition to the lower energy state via stimulated emission of a photon. The probability per unit time for this to happen is Pstim = B21W (!) for some constant B21. • Atoms with energy E2 can also fall back into the lower energy state via spontaneous emission. The probability per unit time for spontaneous emission is independent of W (!). Let's call this probability Pspont = A21 : A21, B21, and B12 are known as Einstein coefficients. a. Write a differential equation for the time dependence of the occupation numbers N1 and N2. -

U.S. Government Printing Office Style Manual, 2008
U.S. Government Printing Offi ce Style Manual An official guide to the form and style of Federal Government printing 2008 PPreliminary-CD.inddreliminary-CD.indd i 33/4/09/4/09 110:18:040:18:04 AAMM Production and Distribution Notes Th is publication was typeset electronically using Helvetica and Minion Pro typefaces. It was printed using vegetable oil-based ink on recycled paper containing 30% post consumer waste. Th e GPO Style Manual will be distributed to libraries in the Federal Depository Library Program. To fi nd a depository library near you, please go to the Federal depository library directory at http://catalog.gpo.gov/fdlpdir/public.jsp. Th e electronic text of this publication is available for public use free of charge at http://www.gpoaccess.gov/stylemanual/index.html. Use of ISBN Prefi x Th is is the offi cial U.S. Government edition of this publication and is herein identifi ed to certify its authenticity. ISBN 978–0–16–081813–4 is for U.S. Government Printing Offi ce offi cial editions only. Th e Superintendent of Documents of the U.S. Government Printing Offi ce requests that any re- printed edition be labeled clearly as a copy of the authentic work, and that a new ISBN be assigned. For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512-1800; DC area (202) 512-1800 Fax: (202) 512-2104 Mail: Stop IDCC, Washington, DC 20402-0001 ISBN 978-0-16-081813-4 (CD) II PPreliminary-CD.inddreliminary-CD.indd iiii 33/4/09/4/09 110:18:050:18:05 AAMM THE UNITED STATES GOVERNMENT PRINTING OFFICE STYLE MANUAL IS PUBLISHED UNDER THE DIRECTION AND AUTHORITY OF THE PUBLIC PRINTER OF THE UNITED STATES Robert C. -

A Steam Issue-48Pp HWM
FROM WATER POWER TO WATT THE ENERGY REVOLUTION OF THE EIGHTEENTH CENTURY Jim Andrew In the seventeenth century, there were no steam engines, no electricity or vehicle fuel from petrol pumps. Power came from human and animal energy, enhanced by wind and water. Changes in water and steam technology in the late-seventeenth and eighteenth centuries contributed to the Industrial Revolution. © Birmingham Museums Trust Sarehole Mill. One of the two remaining water mills in the Birmingham area. 4 www.historywm.com FROM WATER POWER TO WATT ind power, as now, was limited by variations in the strength of the wind, making water the preferred reliable Wsource of significant power for regular activity. The Romans developed water wheels and used them for a variety of activities but the first definitive survey of water power in England was in the Doomsday Book c.1086. At that time, well over five-thousand mills were identified – most were the Norse design with a vertical shaft and horizontal disc of blades. Water-Wheel Technology Traditional water mills, with vertical wheels on horizontal shafts, were well established by the eighteenth century. Many were underpass designs where the water flowed under a wheel with paddles being pushed round by the flow. Over time, many mills adopted designs which had buckets, filled with water at various levels from quite low to the very top of the wheel. The power was extracted by the falling of the water from its entry point to where it was discharged. There was little systematic study of the efficiency of mill-wheel design in extracting energy from the water before the eighteenth century.