Telaprevir (Incivek)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Telaprevir for HIV/Hepatitis C Virus–Coinfected Patients Failing
HIV/AIDS MAJOR ARTICLE Telaprevir for HIV/Hepatitis C Virus–Coinfected Patients Failing Treatment With Pegylated Interferon/Ribavirin (ANRS HC26 TelapreVIH): An Open-Label, Single-Arm, Phase 2 Trial Downloaded from https://academic.oup.com/cid/article/59/12/1768/2895305 by guest on 01 October 2021 Laurent Cotte,1 Joséphine Braun,2 Caroline Lascoux-Combe,3 Corine Vincent,2 Marc-Antoine Valantin,4 Philippe Sogni,5 Karine Lacombe,6 Didier Neau,7 Hugues Aumaitre,8 Dominique Batisse,9 Pierre de Truchis,10 Anne Gervais,11 Christian Michelet,12 Philippe Morlat,13 Daniel Vittecoq,14 Isabelle Rosa,15 Inga Bertucci,16 Stéphane Chevaliez,17 Jean-Pierre Aboulker,2 and Jean-Michel Molina3; for the French National Agency for Research on AIDS and Viral Hepatitis (ANRS) HC26 Study Groupa 1Hospices Civils de Lyon, Croix-Rousse Hospital, and INSERM U1052, 2INSERM SC10-US019, Villejuif, 3Assistance Publique–Hôpitaux de Paris (AP-HP), Saint-Louis Hospital, University of Paris VII Denis Diderot, and Sorbonne Paris-Cité, INSERM U941, 4AP-HP, Pitié-Salpêtrière Hospital, and UMR-S 943, INSERM, 5AP-HP, Cochin Hospital and Paris Descartes University, INSERM U-1016, 6AP-HP, Saint-Antoine Hospital, Sorbonne Universités, UPMC University Paris 06, UMR-S1136, 7Pellegrin University Hospital, Bordeaux, 8Saint-Jean Hospital, Perpignan, 9AP-HP, Georges Pompidou European Hospital, Paris, 10AP-HP, Raymond Poincaré Hospital, Garches, 11AP-HP, Bichat-Claude Bernard Hospital, Paris, 12Pontchaillou University Hospital, Rennes, 13Saint-André University Hospital, Bordeaux, 14AP-HP, Bicètre Hospital, Le Kremlin-Bicètre, 15Créteil Hospital, 16French National Agency for Research on AIDS and Viral Hepatitis, Paris, and 17AP-HP, Henri Mondor Hospital, Créteil, France (See the Editorial Commentary by Rockstroh on pages 1777–8.) Background. -

ARK GENOMIC REVOLUTION MULTI SECTOR ETF (ARKG) HOLDINGS As of 09/27/2021
ARK GENOMIC REVOLUTION MULTI SECTOR ETF (ARKG) HOLDINGS As of 09/27/2021 Company Ticker CUSIP Shares Market Value($) Weight(%) 1 TELADOC HEALTH INC TDOC 87918A105 3,937,797 531,208,815.30 6.99 2 EXACT SCIENCES CORP EXAS 30063P105 3,971,013 381,296,668.26 5.01 3 PACIFIC BIOSCIENCES OF CALIF PACB 69404D108 13,696,148 350,347,465.84 4.61 4 VERTEX PHARMACEUTICALS INC VRTX 92532F100 1,722,281 316,228,014.41 4.16 5 FATE THERAPEUTICS INC FATE 31189P102 4,825,395 312,926,865.75 4.12 6 IONIS PHARMACEUTICALS INC IONS 462222100 8,572,965 310,341,333.00 4.08 7 REGENERON PHARMACEUTICALS REGN 75886F107 430,742 275,201,063.80 3.62 8 TWIST BIOSCIENCE CORP TWST 90184D100 2,237,350 250,829,308.50 3.30 9 TAKEDA PHARMACEUTIC-SP ADR TAK UN 874060205 13,592,076 229,570,163.64 3.02 10 ACCOLADE INC ACCD 00437E102 5,268,242 226,850,500.52 2.98 11 INTELLIA THERAPEUTICS INC NTLA 45826J105 1,508,421 224,965,907.94 2.96 12 VEEVA SYSTEMS INC-CLASS A VEEV 922475108 741,198 222,307,516.14 2.92 13 CAREDX INC CDNA 14167L103 3,433,475 220,978,451.00 2.91 14 CRISPR THERAPEUTICS AG CRSP H17182108 1,804,041 210,044,493.63 2.76 15 INCYTE CORP INCY 45337C102 2,893,385 199,643,565.00 2.63 16 INVITAE CORP NVTA 46185L103 6,059,066 182,135,523.96 2.40 17 ADAPTIVE BIOTECHNOLOGIES ADPT 00650F109 4,888,391 178,377,387.59 2.35 18 BEAM THERAPEUTICS INC BEAM 07373V105 1,849,698 175,110,909.66 2.30 19 SIGNIFY HEALTH INC -CLASS A SGFY 82671G100 8,107,683 160,937,507.55 2.12 20 UIPATH INC - CLASS A PATH 90364P105 2,955,628 155,761,595.60 2.05 21 CASTLE BIOSCIENCES INC CSTL 14843C105 2,130,211 -

Vertex Pharmaceuticals Global Medical Affairs Pharmd Fellowship Program 2020 Message from Global Medical Affairs Leadership
Vertex Pharmaceuticals Global Medical Affairs PharmD Fellowship Program 2020 Message from Global Medical Affairs Leadership Dear Candidates, Vertex Pharmaceuticals in collaboration with Northeastern University is privileged to host and expand the PharmD Fellowship Program. We are excited to work with our fellows as integral members of our team and facilitate in-depth exposure to a range of functional areas, offering them the critical opportunity to gain extensive experience in the biopharmaceutical industry. In addition to fostering each individual’s personal and professional growth, the program’s primary goal is to extend the visibility of valuable contributions that a Doctor of Pharmacy can bring to the pharmaceutical industry. At Vertex, our Global Medical Affairs fellows are highly encouraged to take on immersive projects, allowing them to work across departments with leaders in the industry. We advocate for them to partake in truly unique, impactful work that develops their skills, sparks curiosity, and drives the science. Vertex’s open and innovative culture allows the fellows to ask questions, challenge the status quo, and explore their interests across multiple expertise areas. As a pharmacist, I am extremely proud of the establishment of the Vertex PharmD Fellowship program in Global Medical Affairs and excited to welcome our future fellows. Vertex is an ideal place for fellows to start their professional career, and we are dedicated to the development of the next generation of pharmacist leaders in the biopharmaceutical industry. As Vertex continues to grow and evolve into a company in multiple disease areas, I am confident that our fellows will be an essential part of moving the needle to deliver on our commitment to patients. -

The Legally Binding Text Is the Original French Version TRANSPARENCY
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 14 December 2011 INCIVO 375 mg, film-coated tablet B/4 bottles of 42 tablets (CIP code: 217 378-5) B/1 bottle of 42 tablets (CIP code: 219 249-8) Applicant: JANSSEN-CILAG telaprevir ATC Code: J05AE11 (protease inhibitor) List I Hospital prescription restricted to gastroenterology, hepatology, internal medicine or infectiology specialists. Date of Marketing Authorisation (centralised European procedure): Box of 4 bottles: 19/09/2011 Box of 1 bottle: 13/10/2011 Reason for request: Inclusion on the list of medicines refundable by National Health Insurance and on the list of medicines approved for hospital use. Medical, Economic and Public Health Assessment Division 1/22 1. CHARACTERISTICS OF THE MEDICINAL PRODUCT 1.1. Active ingredient telaprevir 1.2. Background This is an NS3/4A protease inhibitor of the genotype 1 hepatitis C virus. 1.3. Indication “INCIVO, in combination with peginterferon alfa and ribavirin, is indicated for the treatment of genotype 1 chronic hepatitis C in adult patients with compensated liver disease (including cirrhosis): - who are treatment-naive; - who have previously been treated with interferon alfa (pegylated or non-pegylated) alone or in combination with ribavirin, including relapsers, partial responders and null responders (see sections 4.4 and 5.1 of the SPC).” 1.4. Dosage “Treatment with INCIVO must be initiated and monitored by a physician experienced in the management of chronic hepatitis C. INCIVO, 750 mg dose of INCIVO (two 375 mg film-coated tablets) should be taken orally every 8 hours with food (the total daily dose is 6 tablets (2,250 mg)). -

United States Securities and Exchange Commission Form
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event reported): June 17, 2005 VERTEX PHARMACEUTICALS INCORPORATED (Exact name of registrant as specified in its charter) MASSACHUSETTS 000-19319 04-3039129 (State or other jurisdiction of (Commission File Number) (IRS Employer Identification incorporation) No.) 130 Waverly Street Cambridge, Massachusetts 02139 (Address of principal executive offices) (Zip Code) (617) 444-6100 Registrant’s telephone number, including area code: Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below): o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Item 8.01. Other Events. On June 17, 2005, Vertex Pharmaceuticals Incorporated and Merck & Co., Inc. issued a joint press release that announced the initiation of an additional Phase I study with VX-680, a small molecule inhibitor of Aurora kinases. A copy of that press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference. Item 9.01. -

Caracterización Molecular Del Perfil De Resistencias Del Virus De La
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Programa de doctorado en Medicina Departamento de Medicina Facultad de Medicina Universidad Autónoma de Barcelona TESIS DOCTORAL Caracterización molecular del perfil de resistencias del virus de la hepatitis C después del fallo terapéutico a antivirales de acción directa mediante secuenciación masiva Tesis para optar al grado de doctor de Qian Chen Directores de la Tesis Dr. Josep Quer Sivila Dra. Celia Perales Viejo Dr. Josep Gregori i Font Laboratorio de Enfermedades Hepáticas - Hepatitis Víricas Vall d’Hebron Institut de Recerca (VHIR) Barcelona, 2018 ABREVIACIONES Abreviaciones ADN: Ácido desoxirribonucleico AK: Adenosina quinasa ALT: Alanina aminotransferasa ARN: Ácido ribonucleico ASV: Asunaprevir BOC: Boceprevir CCD: Charge Coupled Device CLDN1: Claudina-1 CHC: Carcinoma hepatocelular DAA: Antiviral de acción directa DC-SIGN: Dendritic cell-specific ICAM-3 grabbing non-integrin DCV: Daclatasvir DSV: Dasabuvir -
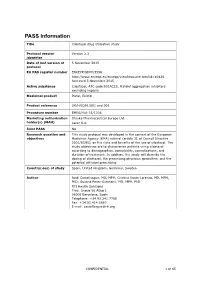
PASS Information
PASS Information Title Cilostazol drug utilisation study Protocol version Version 2.3 identifier Date of last version of 5 November 2015 protocol EU PAS register number ENCEPP/SDPP/3596 http://www.encepp.eu/encepp/viewResource.htm?id=10426 Accessed 5 November 2015 Active substance Cilostazol, ATC code B01AC23, Platelet aggregation inhibitors excluding heparin Medicinal product Pletal, Ekistol Product reference UK/H/0291/001 and 002 Procedure number EMEA/H/A-31/1306 Marketing authorisation Otsuka Pharmaceutical Europe Ltd. holder(s) (MAH) Lacer S.A. Joint PASS No Research question and This study protocol was developed in the context of the European objectives Medicines Agency (EMA) referral (article 31 of Council Directive 2001/83/EC) on the risks and benefits of the use of cilostazol. The study objectives are to characterise patients using cilostazol according to demographics, comorbidity, comedications, and duration of treatment. In addition, the study will describe the dosing of cilostazol, the prescribing physician specialties, and the potential off-label prescribing. Country(-ies) of study Spain, United Kingdom, Germany, Sweden Author Jordi Castellsague, MD, MPH; Cristina Varas-Lorenzo, MD, MPH, PhD; Susana Perez-Gutthann, MD, MPH, PhD RTI Health Solutions Trav. Gracia 56 Atico 1 08006 Barcelona, Spain Telephone: +34.93.241.7766 Fax: +34.93.414.2610 E-mail: [email protected] CONFIDENTIAL 1 of 65 Marketing Authorisation Holder(s) Marketing authorisation Otsuka Pharmaceutical Europe Ltd. holder(s) Gallions Wexham Springs Framewood Road Wexham SL3 6PJ, UK Lacer S.A. Sardenya 350 08025 Barcelona Spain MAH contact person Dr Marco Avila Regional Vice President, Medical Europe Otsuka Pharmaceutical Europe Ltd EU QPPV Dr Achint Kumar Otsuka Europe Development and Commercialisation Limited (OEDC) Head of Medical Dr Sanjay Kapoor Compliance-Europe Otsuka Pharmaceutical Europe Ltd. -

Medicinal Product No Longer Authorised
Assessment report INCIVO telaprevir Procedure No.: EMEA/H/C/002313/ Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Medicinal product no longer authorised 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8613 E-mail [email protected] Website www.ema.europaeu An agency of the European Union © European Medicines Agency, 2011. Reproduction is authorised provided the source is acknowledged. 21 July 2011 EMA/CHMP/475470/2011 Committee for Medicinal Products for Human Use (CHMP) CHMP assessment report INCIVO International non-proprietary name: telaprevir authorised Procedure No. EMEA/H/C/002313 longer no product Medicinal 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8613 E-mail [email protected] Website www.ema.europaeu An agency of the European Union CHMP assessment report Page 2/109 Product information Name of the medicinal product: INCIVO Applicant: Janssen-Cilag International N.V. Turnhoutseweg 30 BE-2340 Beerse Belgium Active substance: telaprevir International Nonproprietary Name/Common Name: telaprevir Pharmaco-therapeutic group Protease inhibitors authorised (ATC Code): (J05AE) INCIVO, in combination with peginterferon alfa and ribavirin, is indicated for the treatment of genotype Therapeutic indication(s): 1 chronic hepatitis C in adult patients with compensated liver disease (including cirrhosis): - who are treatment-naïve; - who have previously been treated with interferonlonger alfa (pegylated or non-pegylated) alone or in combination with ribavirin, including relapsers, partial responders and null responders (see sections 4.4 and 5.1). -

PASS Information Title Cilostazol Drug Utilisation Study
PASS Information Title Cilostazol drug utilisation study Protocol version Version 2 identifier Date of last version of February 28, 2013 protocol EU PAS register number Registration is planned prior to study initiation once the protocol is final and has received regulatory endorsement by the Committee for Medicinal Products for Human Use (CHMP) Active substance Cilostazol, ATC code B01AC23, Platelet aggregation inhibitors excluding heparin Medicinal product Pletal, Ekistol Product reference UK/H/0291/001 and 002 Procedure number EMEA/H/A-31/1306 Marketing authorisation Otsuka Pharmaceutical Europe Ltd. holder(s) (MAH) Lacer S.A. Joint PASS No Research question and This study protocol was developed in the context of the European objectives Medicines Agency (EMA) referral (article 31 of Council Directive 2001/83/EC) on the risks and benefits of the use of cilostazol. The study objectives are to characterise patients using cilostazol according to demographics, comorbidity, comedications, and duration of treatment. In addition, the study will describe the dosing of cilostazol, the prescribing physician specialties, and the potential off-label prescribing. Country(-ies) of study Spain, United Kingdom, Germany, Sweden Author Jordi Castellsague, MD, MPH; Cristina Varas-Lorenzo, MD, MPH, PhD; Susana Perez-Gutthann, MD, MPH, PhD RTI Health Solutions Trav. Gracia 56 Atico 1 08006 Barcelona, Spain Telephone: +34.93.241.7766 Fax: +34.93.414.2610 E-mail: [email protected] CONFIDENTIAL 1 of 56 Marketing Authorisation Holder(s) Marketing authorisation -

Pax Large Cap Fund USD 7/31/2021 Port
Pax Large Cap Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight Microsoft Corporation 89,240,649.84 6.2 Apple Inc. 56,474,074.80 3.9 Alphabet Inc. Class A 45,168,406.39 3.1 Applied Materials, Inc. 40,802,748.42 2.8 United Parcel Service, Inc. Class B 40,145,031.68 2.8 Amazon.com, Inc. 40,024,252.52 2.8 Procter & Gamble Company 38,175,527.61 2.6 Alphabet Inc. Class C 37,288,542.96 2.6 T-Mobile US, Inc. 36,913,478.16 2.5 CVS Health Corporation 35,823,305.60 2.5 Bristol-Myers Squibb Company 35,683,263.33 2.5 Trane Technologies plc 34,492,144.83 2.4 Voya Financial, Inc. 34,364,677.20 2.4 Fiserv, Inc. 33,109,434.63 2.3 Medtronic Plc 32,631,060.24 2.2 Lowe's Companies, Inc. 32,345,328.78 2.2 JPMorgan Chase & Co. 31,275,786.80 2.2 salesforce.com, inc. 31,164,938.74 2.1 Aptiv PLC 30,049,685.00 2.1 Target Corporation 29,624,476.10 2.0 Becton, Dickinson and Company 29,334,525.00 2.0 Citizens Financial Group, Inc. 28,825,635.20 2.0 Merck & Co., Inc. 28,216,517.16 1.9 BlackRock, Inc. 28,142,268.01 1.9 Vertex Pharmaceuticals Incorporated 26,265,874.00 1.8 Lincoln National Corporation 25,842,318.84 1.8 Sysco Corporation 25,428,711.00 1.8 Equinix, Inc. -

Inhibitor Development Against P7 Channel in Hepatitis C Virus
molecules Article Inhibitor Development against p7 Channel in Hepatitis C Virus Shukun Wei 1,2,†, Xiaoyou Hu 2,3,†, Lingyu Du 1, Linlin Zhao 1,4, Hongjuan Xue 5, Chaolun Liu 6, James J. Chou 4, Jin Zhong 2,3,6, Yimin Tong 3,*, Shuqing Wang 7,* and Bo OuYang 1,2,* 1 State Key Laboratory of Molecular Biology, Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 333 Haike Road, Shanghai 201203, China; [email protected] (S.W.); [email protected] (L.D.); [email protected] (L.Z.) 2 University of Chinese Academy of Sciences, Beijing 100049, China; [email protected] (X.H.); [email protected] (J.Z.) 3 CAS Key Laboratory of Molecular Virology and Immunology, Institute Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China 4 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA; [email protected] 5 National Facility for Protein Science in Shanghai, ZhangJiang Lab, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China; [email protected] 6 ShanghaiTech University, Shanghai 201210, China; [email protected] 7 School of Pharmacy, Tianjin Medical University, Tianjin 300070, China * Correspondence: [email protected] (Y.T.); [email protected] (S.W.); [email protected] (B.O.) † This authors contributed equally. Abstract: Hepatitis C Virus (HCV) is the key cause of chronic and severe liver diseases. The recent direct-acting antiviral agents have shown the clinical success on HCV-related diseases, but the rapid HCV mutations of the virus highlight the sustaining necessity to develop new drugs. -

PD2M Newsletter
PD2M Newsletter March 2020 Note from the Editor Our first issue of the PD2M Newsletter 2020 highlights two interesting topics: - Pharma 4.0: The term “Industry 4.0” was first used in Germany to incentivize modernization of manufacturing. The International Society for Pharmaceutical Engineering (ISPE) adapted it 2017 and the concept of “Pharma 4.0” was born. The Carla Luciani vision of highly efficient, vastly automated, self-driven manufacturing processes is extremely appealing to Pharmaceutical Discovery, practitioners. But separating hype from reality is important. Get Development and Manufacturing a feel of what is going on from Nima Yazdanpanah Forum, Newsletter Chair (Procegence), Shujauddin Changi (Vertex), Moiz Diwan (Abbvie), and Christopher Burcham (Eli Lilly) who reported RNA-Tx, Lilly Research Pharma 4.0 Highlights from the last AIChE Annual Meeting. Laboratories, Eli Lilly & Co. - Connect @ AIChE: Meet candidates in a poster session & reception at the 2020 AIChE Annual Meeting in San Francisco. If you are a graduate student/postdoc who are available for In this issue: employment by summer 2021 or you work in industry and want to meet new talent and grow connections, this is a great event Note from the Page 1 to do so. Learn everything you need to know here. Editor There is still more to come on PD2M programming and activities. Don’t Pharma 4.0 Page 2 miss future issues of the PD2M Newsletter. Highlights Connect @ AIChE Page 6 It is all about connectivity… 1 | Page Page | 1 Page | 1 PD2M Newsletter Pharma 4.0 Highlights from AIChE Annual Meeting 2019 The Pharma 4.0 paradigm, analogous to the The Pharma 4.0 was topic of two plenary sessions Industry 4.0, intends to utilize enabling technologies in the AIChE Annual meeting 2019 in Orlando, FL, in the pharmaceutical and biopharmaceutical held by PD2M and Next-Gen Manufacturing industries.