Resource Use Strategies of Wading Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Handling and Intake of Plastic Debris by Wood Storks at an Urban Site in South-Eastern Brazil: Possible Causes and Consequences
NORTH-WESTERN JOURNAL OF ZOOLOGY 11 (2): 372-374 ©NwjZ, Oradea, Romania, 2015 Article No.: 147601 http://biozoojournals.ro/nwjz/index.html Handling and intake of plastic debris by Wood Storks at an urban site in South-eastern Brazil: possible causes and consequences Ivan SAZIMA1,* and Giulia B. D’ANGELO2 1. Museu de Zoologia, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil. 2. Instituto de Biologia, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil. *Corresponding author, I. Sazima, E-mail: [email protected] Received: 20. September 2014 / Accepted: 12. December 2014 / Available online: 01. November 2015 / Printed: December 2015 Anthropogenic inedible debris increases in the picked pieces of plastic cable were individually identified food chain of seabirds, marine turtles, and by differences on neck feathering and bill colour. cetaceans and becomes a worldwide problem (e.g. Sequenced photographs allowed the timing of the behavioural events for two of these individuals. Denuncio et al. 2011, Avery-Gomm et al. 2013, Throughout the observations, we used the “ad libitum” Schluyter et al. 2013, Donnelly-Greenan et al. 2014, sampling (Altmann 1974), which is adequate to record Provencher et al. 2015), but this material is rarely rare events. reported entering the food chain of inland wildlife (Peris 2003, Booth 2011, Henry et al. 2011). Storks Among small groups of Wood Storks that varied of the genus Ciconia are opportunistic visual from 2 to 8 migrant individuals comprised of up to hunters that prey mostly on fish, frogs, snakes, 2 adults and 6 juveniles, we recorded the small mammals, and a variety of insects (Elliott behaviour of 4 juveniles (subadults sensu Coulton 1992). -
Dwarf Thistle, Cirsi
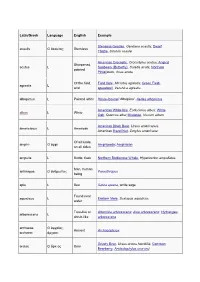
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Wood-Stork-2005.Pdf
WOOD STORK Mycteria americana Photo of adult wood stork in landing posture and chicks in Close-up photo of adult wood stork. nest. Photo courtesy of USFWS/Photo by George Gentry Photo courtesy of U.S. Army Corps of Engineers. FAMILY: Ciconiidae STATUS: Endangered - U.S. Breeding Population (Federal Register, February 28, 1984) [Service Proposed for status upgrade to Threatened, December 26, 2013] DESCRIPTION: Wood storks are large, long-legged wading birds, about 45 inches tall, with a wingspan of 60 to 65 inches. The plumage is white except for black primaries and secondaries and a short black tail. The head and neck are largely unfeathered and dark gray in color. The bill is black, thick at the base, and slightly decurved. Immature birds have dingy gray feathers on their head and a yellowish bill. FEEDING HABITS: Small fish from 1 to 6 inches long, especially topminnows and sunfish, provide this bird's primary diet. Wood storks capture their prey by a specialized technique known as grope-feeding or tacto-location. Feeding often occurs in water 6 to 10 inches deep, where a stork probes with the bill partly open. When a fish touches the bill it quickly snaps shut. The average response time of this reflex is 25 milliseconds, making it one of the fastest reflexes known in vertebrates. Wood storks use thermals to soar as far as 80 miles from nesting to feeding areas. Since thermals do not form in early morning, wood storks may arrive at feeding areas later than other wading bird species such as herons. -

Spread-Wing Postures and Their Possible Functions in the Ciconiidae
THE AUK A QUARTERLY JOURNAL OF ORNITHOLOGY Von. 88 Oc:roBE'a 1971 No. 4 SPREAD-WING POSTURES AND THEIR POSSIBLE FUNCTIONS IN THE CICONIIDAE M. P. KAI-IL IN two recent papers Clark (19'69) and Curry-Lindahl (1970) have reported spread-wingpostures in storks and other birds and discussed someof the functionsthat they may serve. During recent field studies (1959-69) of all 17 speciesof storks, I have had opportunitiesto observespread-wing postures. in a number of speciesand under different environmentalconditions (Table i). The contextsin which thesepostures occur shed somelight on their possible functions. TYPES OF SPREAD-WING POSTURES Varying degreesof wing spreadingare shownby at least 13 species of storksunder different conditions.In somestorks (e.g. Ciconia nigra, Euxenuragaleata, Ephippiorhynchus senegalensis, and ]abiru mycteria) I observedno spread-wingpostures and have foundno referenceto them in the literature. In the White Stork (Ciconia ciconia) I observedonly a wing-droopingposture--with the wings held a short distanceaway from the sidesand the primaries fanned downward--in migrant birds wetted by a heavy rain at NgorongoroCrater, Tanzania. Other species often openedthe wingsonly part way, in a delta-wingposture (Frontis- piece), in which the forearmsare openedbut the primariesremain folded so that their tips crossin front o.f or below the. tail. In some species (e.g. Ibis leucocephalus)this was the most commonly observedspread- wing posture. All those specieslisted in Table i, with the exception of C. ciconia,at times adopted a full-spreadposture (Figures i, 2, 3), similar to those referred to by Clark (1969) and Curry-Lindahl (1970) in severalgroups of water birds. -

The History of the Southern Florida Wood Stork Population
Wilson Bull., 98(3), 1986, pp. 368-386 THE HISTORY OF THE SOUTHERN FLORIDA WOOD STORK POPULATION JAMES A. KUSHLAN AND PAULA C. FROHRING ’ AswnAcr.-The largest segment of the North American Wood Stork (Mycteria ameri- cam) population traditionally nested in southern Florida, where its perceived population decrease over the last 50 years resulted in its addition to the Federal endangered species list. Previously published reports placed the southern Florida population at 30,000 to 100,000 storks. A complete search of published and unpublished records of Wood Storks nesting in southern Florida, however, failed to support such a long-term population decrease. We cannot demonstrate that the historical population was any larger than it was in 1967, when there were 9400 pairs. The Corkscrew-Big Cypress nesting group, not that of the Everglades, was historically the most numerically important population segment. The historic nesting location for southernmost Wood Storks was not inland in the Everglades but on Cape Sable, which has been subject to the effects of drainage canals. The population size of several decades ago is irrelevant to current conservation strategy because the southern Florida marshes have been irrevocably altered reducing their ability to support storks. We can document a population decrease of 75% from 1967 to 1981-82 in southern Florida, a time frame coincident with the operation of water management policies in the Everglades. Wood Storks recently have begun abandoning traditional colony sites in Everglades National Park in favor of sites in shallow reservoirs to the north. Drainage of the Big Cypress Swamp and maintenance of seasonally excessive water levels in the Everglades of Everglades National Park account for the storks’ repeated nesting failure and population decrease. -

Factors Affecting Reproductive Success of Wood Storks (Mycteria Americana) in East-Central Georgia
The Auk 112(1):237-243, 1995 FACTORS AFFECTING REPRODUCTIVE SUCCESS OF WOOD STORKS (MYCTERIA AMERICANA) IN EAST-CENTRAL GEORGIA MALCOLM C. COULTER• AND A. LAWRENCEBRYAN, JR. SavannahRiver Ecology Laboratory, Drawer E, Aiken,South Carolina 29802, USA ABS?R•cr.--From1984 through 1989, we examinedthe reproductivesuccess of WoodStorks (Mycteriaamericana) at the Birdsvillecolony in east-centralGeorgia. Average fledging success rangedamong years from 0.33to 2.16fledglings per nest.For neststhat producedfledglings, prey availabilitywas an importantfactor affecting reproductive success. Yearly averageprey densitiesat foragingsites were significantlycorrelated with the averagenumber of fledglings producedfrom successfulnests. Among 243 nestsobserved, all eggsor chickswere lostfrom 104(43%) nests. Five factorswere associatedwith the lossof entire clutchesor broods.During the two driest years, 1985 and 1988, raccoon(Procyon lotor) predation eliminated almost all chicks.Many nestswere abandonedearly in 1989, following periodsof cold weather when the parentsappeared to be under stress.In 1985, the birds desertedthe colony before egg laying when the area experiencedfreezing weather. Following nest abandonmentswithin the colony,paired adultsthat presumablyhad abandonedtheir nestswere involved in nest takeoversthat alsocaused the lossof eggsand chicks.Three stormsduring the studycaused the lossof a few nests.Some losses were due to unknown factors.The importanceof these mortality factorsvaried from year to year. Nest abandonmentsand subsequentaggression seemto be related to cold periodsearly in the season.Raccoon predation seems to be related to drying out of the water under the colony.This suggeststhat the storkshave a window in time when it is bestto breed--afterthe winter and earlyspring cold weather and beforethe water dries under the colony in the summer.Received 4 December1991, accepted 15 November 1992. -

Recent Wood Stork Population Trends in the United States
Wilson’ Bull., 91(4), 1979, pp. 512-523 RECENT WOOD STORK POPULATION TRENDS IN THE UNITED STATES JOHN C. OGDEN AND STEPHEN A. NESBITT The population of Wood Storks (Mycteria americana) resident in the United States occurs on the coastal plain of the Gulf of Mexico and the southern Atlantic states. Historically, storks nested in all coastal states from Texas to South Carolina (Bent 1926, Cone and Hall 1970, Dusi and Dusi 1968, Howell 1932, Oberholser 1938, Oberholser and Kincaid 1974, Wayne 1910), although colonies outside Florida formed irregularly and contained few birds. The United States population of storks was not greatly disturbed during the plume-hunting era (Allen 1958), and probably con- tained between 75,000 and 100,000 birds of all age classes during the early twentieth century (Ogden 1978). I ncreased land development and the as- sociated drainage of freshwater wetlands eliminated many nesting and feeding sites, resulting in a severe decline in the total number of storks. Concern for the fate of this species in the United States was first expressed during the late 1950s (Allen 1958, Sprunt and Kahl 1960). A series of aerial surveys was conducted between 1957 and 1960, in an attempt to locate all remaining Wood Stork nesting colonies in the United States. Renewed concern for the status of storks during the early 1970s resulted in a second series of aerial surveys beginning in 1974. These 2 surveys produced the first complete counts of the number of storks nesting in the United States, and revealed that major colonies continued to decline between surveys. -

Plumage and Behavioral Development of Nestling White Ibises
Wilson Bull., 102(2), 1990, pp. 226-238 PLUMAGE AND BEHAVIORAL DEVELOPMENT OF NESTLING WHITE IBISES TONI L. DE SANTO,’ SUSAN G. MCDOWELL,~ AND KEITH L. BILDSTEIN~ Ans-rticr.- We describe the physical characteristicsand behavioral development OI 17 hand-reared and more than 400 parent-reared nestling White Ibises (Eudocimus albus) hatched in 1985 through 1988 at Pumpkinseed Island, a large colony site in coastal South Carolina. Hatchling ibises are covered with a Pale Neutral Gray to Jet Black natal plumage. About 30% of the hatchlingspossess a tuft of white feathers on their crown, and this pattern persiststhroughout the nestling period. Juvenal plumage, which is complete by 60 days, is mainly Vandyke Brown and Blackish Neutral Gray dorsally and creamy white ventrally. The bill, which is straight at hatching, begins to curve downward at about 14 days. Nestling White Ibises exhibit considerableindividual variation in bill markings from approximately 10 days of age through fledging. Increasingly persistent beggingvocalizations begin within hours of hatching. Nestlings walk on partially extended legsat eight days of age, pirate food from other nestlingsand form crechesat 2 1 days of age, and fledge and join all juvenile and mixed-age feeding flocks at 45-55 days of age. We suggestthat the phenotypic variability in plumage, bill coloration, and beggingcalls we describeenables parental ibises to identify more easily their offspringat the colony site. Received27 Feb. 1989, accepted12 Nov. 1989. Although the plumage and behavioral development of several species of wading birds has been studied in considerable detail (e.g., Hammerkops [&opus umbretta], Wilson et al. 1988; storks, Kahl 1962, 1966; Thomas 1984; herons, Gross 1923; Gavin0 and Dickerman 1972; Juarez and Dickerman 1972; Mc Vaugh 1972,1975; Snow 1974; Merritt 198 l), there are few detailed studies of juvenile ibises. -

THE BRITISH LIST the Official List of Bird Species Recorded in Britain Sponsored by Leica Sports Optics
THE BRITISH LIST The official list of bird species recorded in Britain sponsored by Leica Sports Optics CATEGORY E SPECIES Category E comprises those species that have been recorded as introductions, human-assisted transportees or escapees from captivity, whose breeding populations (if any) are thought not to be self-sustaining. Species in Category E that have bred in the wild in Britain are designated as E*. Species listed in Category E form no part of the British List, unless they are also included in Categories A, B or C. Descriptions and definitions of Categories of the British List can be found at BOU (2017)19. Category E has been compiled largely from county/regional bird reports and other published listings, and is not exhaustive. BOURC is unable to confirm the correct identification of species in Category E, or the circumstances in which they were recorded. Doubtful cases have been excluded, pending enquiries, along with species not recorded since 1 January 1950. The BOU is responsible for maintaining the British List. Part of this responsibility is to monitor the occurrence of non-native species which may qualify for addition to Category C of the British List. To undertake this we require published information from which to work and which we can quote as reference. The BOU encourages observers to report records of non-native species to the relevant local bird recorder. Local recorders and those producing local, county or regional publications are encouraged to publish these records, and to make them available to the Rare Breeding Birds Panel (RBBP) who publish annual reports1- 7,9,10,12 of non-native species breeding in Britain in British Birds. -

Sacred Ibis (Threskiornis Aethiopicus)1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. WEC267 Florida's Introduced Birds: Sacred Ibis (Threskiornis aethiopicus)1 Steve Johnson and Monica McGarrity2 Many non-native birds have been introduced in prey. Sacred Ibises are larger than Florida's native Florida—perhaps as many as 200 species! Of these, ibises. Adults are approximately 30 inches long (75 at least 16 introduced species are considered cm) with a wingspan of 44–49 inches (112–124 established, according to various authorities, and cm) and weigh about 3 pounds (1.4 kg). Sacred Ibises some are now considered invasive and could have (Fig. 1) have mostly white bodies and wings; the serious impacts in Florida. This fact sheet introduces trailing edges of their wings (tips of the feathers) are the Sacred Ibis, and is one of a series of fact sheets gray-black. They have very distinctive long, black about Florida's established non-native birds and their feathers or plumes on their rumps (Fig. 1). During the impacts on our native ecosystems, economy, and the breeding season the feathers on the sides of their quality of life of Floridians. For more information on chests and on the outer wings (near the edge when Florida's introduced birds, how they got here, and the folded) may have a yellowish (or reddish) tinge, and problems they cause, read "Florida's Introduced their lower legs may be tinged with reddish-copper; Birds: An Overview" bare patches of scarlet-red skin may also be visible (http://edis.ifas.ufl.edu/UW297) and the other fact under their wings. -

Consequences of Nesting Date on Nesting Success and Juvenile Survival in White Ibis
CONSEQUENCES OF NESTING DATE ON NESTING SUCCESS AND JUVENILE SURVIVAL IN WHITE IBIS By JOHN DAVID SEMONES A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 Copyright 2003 by John David Semones ACKNOWLEDGMENTS This project succeeded in part due to the support and physical aid of numerous individuals and agencies. First, I would like to thank all the field technicians, volunteers, and birdwatchers across the state of Florida and those who wrote to me with roost information from Louisiana. These individuals provided invaluable aid in data collection, monitoring of roost locations, and information on lost birds. My advisor, Peter Frederick, and members of his research group, Becky Hylton and Julie Heath, provided continuous input on the project’s design and implementation, as well as support in the field. Peter, in particular, kept me pointed in the right direction and taught me the correct time and place for using the word “indubitably.” His knowledge and enthusiasm about wading birds and the Everglades are contagious and I now know more about engine mechanics than most people rightfully should know. Throughout this process my committee members, Ken Meyer and Madan Oli, provided feedback on my ideas and helped make this a well-rounded project. Ken’s advice on telemetry flights and radio- tracking methods was particularly instrumental. I am grateful to A.R.M. Loxahatchee National Wildlife Refuge, Everglades National Park, and the Palm Beach County Waste Facility for permission to conduct this research with unfettered access to these locations. -

Internest Displacement of White Ibis Eggs
SHORT COMMUNICATIONS 273 of the Fitzpatrick Institutes’ 25th Anniversary Expedition to Chile. Financial support was received from the South African CSIR and the University of Cape Town. LITERATURE CITED CHAPMAN, S. E. 1973. The grey gull, Lams modestus.Sea Swallow 22:7-10. DUFFY, D. C. 1980. Patterns of piracy by Peruvian seabirds: a depth hypothesis. Ibis 122: 521-525. -. 1983. The foraging ecology of Peruvian seabirds. Auk 100:800-810. HARRISON, P. 1983. Seabirds: an identification guide. Croom Helm, Beckenham, England. HOWELL, T. R., B. ARAYA, AND W. R. MILLIE. 1974. Breeding biology of the gray gull Lams modestus.Univ. Calif. Publ. Zool. 104:1-57. JOHNSON,A. W. 1965. Birds of Chile. Vol. II. Platt Estab. Graficos, Buenos Aires, Argen- tina. MURPHY, R. C. 1936. Oceanic birds of South America. Macmillan, New York, New York. SEARCY,W. A. 1978. Foraging successin three age classes of Glaucous-winged Gulls. Auk 95:586-590. VERBEEK, N. A. M. 1977. Comparative feeding ecology of adult and immature Herring Gulls. Wilson Bull. 89:4 15-42 1. P. G. RYAN, P. A. R. HOCKEY, AND A. L. BOSMAN, Fitzpatrick Inst., Univ. Cape Town, Rondebosch7700, South Africa. ReceivedI7 June 1986. accepted6 Oct. 1986. WilsonBull., 99(2), 1987, pp. 273-275 Internest displacement of White Ibis eggs.-During a study of nesting success at a White Ibis (Eudocimusa/bus) colony (Shields and Pamell 1986), I observed five cases in which an egg laid in one nest was subsequently found in another nest in the same tree or shrub. The study was conducted during the 1983 and 1984 breeding seasons at Battery Island, North Carolina (33”54N,’ 78”olW),’ where White Ibises nested in a maritime shrub thicket (see Shields and Pamell 1986 for a complete description of the area).