Lepidoptera: Sphingidae) from Seychelles, with a Description of a New Insular Subspecies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fung Yuen SSSI & Butterfly Reserve Moth Survey 2009
Fung Yuen SSSI & Butterfly Reserve Moth Survey 2009 Fauna Conservation Department Kadoorie Farm & Botanic Garden 29 June 2010 Kadoorie Farm and Botanic Garden Publication Series: No 6 Fung Yuen SSSI & Butterfly Reserve moth survey 2009 Fung Yuen SSSI & Butterfly Reserve Moth Survey 2009 Executive Summary The objective of this survey was to generate a moth species list for the Butterfly Reserve and Site of Special Scientific Interest [SSSI] at Fung Yuen, Tai Po, Hong Kong. The survey came about following a request from Tai Po Environmental Association. Recording, using ultraviolet light sources and live traps in four sub-sites, took place on the evenings of 24 April and 16 October 2009. In total, 825 moths representing 352 species were recorded. Of the species recorded, 3 meet IUCN Red List criteria for threatened species in one of the three main categories “Critically Endangered” (one species), “Endangered” (one species) and “Vulnerable” (one species” and a further 13 species meet “Near Threatened” criteria. Twelve of the species recorded are currently only known from Hong Kong, all are within one of the four IUCN threatened or near threatened categories listed. Seven species are recorded from Hong Kong for the first time. The moth assemblages recorded are typical of human disturbed forest, feng shui woods and orchards, with a relatively low Geometridae component, and includes a small number of species normally associated with agriculture and open habitats that were found in the SSSI site. Comparisons showed that each sub-site had a substantially different assemblage of species, thus the site as a whole should retain the mosaic of micro-habitats in order to maintain the high moth species richness observed. -

Boophone Disticha
Micropropagation and pharmacological evaluation of Boophone disticha Lee Cheesman Submitted in fulfilment of the academic requirements for the degree of Doctor of Philosophy Research Centre for Plant Growth and Development School of Life Sciences University of KwaZulu-Natal, Pietermaritzburg April 2013 COLLEGE OF AGRICULTURE, ENGINEERING AND SCIENCES DECLARATION 1 – PLAGIARISM I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research contained in this thesis, except where otherwise indicated, is my original research. 2. This thesis has not been submitted for any degree or examination at any other University. 3. This thesis does not contain other persons’ data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. 4. This thesis does not contain other persons’ writing, unless specifically acknowledged as being sourced from other researchers. Where other written sources have been quoted, then: a. Their words have been re-written but the general information attributed to them has been referenced. b. Where their exact words have been used, then their writing has been placed in italics and inside quotation marks, and referenced. 5. This thesis does not contain text, graphics or tables copied and pasted from the internet, unless specifically acknowledged, and the source being detailed in the thesis and in the reference section. Signed at………………………………....on the.....….. day of ……......……….2013 ______________________________ SIGNATURE i STUDENT DECLARATION Micropropagation and pharmacological evaluation of Boophone disticha I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research reported in this dissertation, except where otherwise indicated is the result of my own endeavours in the Research Centre for Plant Growth and Development, School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg. -

Western Ghats), Idukki District, Kerala, India
International Journal of Entomology Research International Journal of Entomology Research ISSN: 2455-4758 Impact Factor: RJIF 5.24 www.entomologyjournals.com Volume 3; Issue 2; March 2018; Page No. 114-120 The moths (Lepidoptera: Heterocera) of vagamon hills (Western Ghats), Idukki district, Kerala, India Pratheesh Mathew, Sekar Anand, Kuppusamy Sivasankaran, Savarimuthu Ignacimuthu* Entomology Research Institute, Loyola College, University of Madras, Chennai, Tamil Nadu, India Abstract The present study was conducted at Vagamon hill station to evaluate the biodiversity of moths. During the present study, a total of 675 moth specimens were collected from the study area which represented 112 species from 16 families and eight super families. Though much of the species has been reported earlier from other parts of India, 15 species were first records for the state of Kerala. The highest species richness was shown by the family Erebidae and the least by the families Lasiocampidae, Uraniidae, Notodontidae, Pyralidae, Yponomeutidae, Zygaenidae and Hepialidae with one species each. The results of this preliminary study are promising; it sheds light on the unknown biodiversity of Vagamon hills which needs to be strengthened through comprehensive future surveys. Keywords: fauna, lepidoptera, biodiversity, vagamon, Western Ghats, Kerala 1. Introduction Ghats stretches from 8° N to 22° N. Due to increasing Arthropods are considered as the most successful animal anthropogenic activities the montane grasslands and adjacent group which consists of more than two-third of all animal forests face several threats (Pramod et al. 1997) [20]. With a species on earth. Class Insecta comprise about 90% of tropical wide array of bioclimatic and topographic conditions, the forest biomass (Fatimah & Catherine 2002) [10]. -

Wildlife Review Cover Image: Hedgehog by Keith Kirk
Dumfries & Galloway Wildlife Review Cover Image: Hedgehog by Keith Kirk. Keith is a former Dumfries & Galloway Council ranger and now helps to run Nocturnal Wildlife Tours based in Castle Douglas. The tours use a specially prepared night tours vehicle, complete with external mounted thermal camera and internal viewing screens. Each participant also has their own state- of-the-art thermal imaging device to use for the duration of the tour. This allows participants to detect animals as small as rabbits at up to 300 metres away or get close enough to see Badgers and Roe Deer going about their nightly routine without them knowing you’re there. For further information visit www.wildlifetours.co.uk email [email protected] or telephone 07483 131791 Contributing photographers p2 Small White butterfly © Ian Findlay, p4 Colvend coast ©Mark Pollitt, p5 Bittersweet © northeastwildlife.co.uk, Wildflower grassland ©Mark Pollitt, p6 Oblong Woodsia planting © National Trust for Scotland, Oblong Woodsia © Chris Miles, p8 Birdwatching © castigatio/Shutterstock, p9 Hedgehog in grass © northeastwildlife.co.uk, Hedgehog in leaves © Mark Bridger/Shutterstock, Hedgehog dropping © northeastwildlife.co.uk, p10 Cetacean watch at Mull of Galloway © DGERC, p11 Common Carder Bee © Bob Fitzsimmons, p12 Black Grouse confrontation © Sergey Uryadnikov/Shutterstock, p13 Black Grouse male ©Sergey Uryadnikov/Shutterstock, Female Black Grouse in flight © northeastwildlife.co.uk, Common Pipistrelle bat © Steven Farhall/ Shutterstock, p14 White Ermine © Mark Pollitt, -

Scaevola Taccada (Gaertn.) Roxb
BioInvasions Records (2021) Volume 10, Issue 2: 425–435 CORRECTED PROOF Rapid Communication First record of naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) in southeastern Mexico Gonzalo Castillo-Campos1,*, José G. García-Franco2 and M. Luisa Martínez2 1Red de Biodiversidad y Sistemática, Instituto de Ecología, A.C., Xalapa, Veracruz, 91073, México 2Red de Ecología Funcional, Instituto de Ecología, A.C. Xalapa, Veracruz, 91073, México Author e-mails: [email protected] (GCC), [email protected] (JGGF), [email protected] (MLM) *Corresponding author Citation: Castillo-Campos G, García- Franco JG, Martínez ML (2021) First Abstract record of naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) Scaevola taccada (Gaertn.) Roxb. is native of Asia and eastern Africa but has been in southeastern Mexico. BioInvasions introduced into the Americas as an ornamental urban plant. This paper reports, for Records 10(2): 425–435, https://doi.org/10. the first time, the presence of Scaevola taccada in natural environments from 3391/bir.2021.10.2.21 southeastern Mexico. Several populations of S. taccada were identified during a Received: 23 July 2020 botanical survey of the coastal dunes of the Cozumel Island Biosphere Reserve Accepted: 22 October 2020 (State of Quintana Roo, Mexico) aimed at recording the most common plant Published: 22 January 2021 species. Scaevola taccada is considered as an invasive species of coastal areas in this region. Evidence of its invasiveness is suggested by the fact that populations Handling editor: Oliver Pescott consisting of individuals of different size classes are found distributed throughout Thematic editor: Stelios Katsanevakis the island. Furthermore, they appear to belong to different generations since we Copyright: © Castillo-Campos et al. -

Phyllosticta Hymenocallidicola Fungal Planet Description Sheets 139
138 Persoonia – Volume 27, 2011 Phyllosticta hymenocallidicola Fungal Planet description sheets 139 Fungal Planet 95 – 6 December 2011 Phyllosticta hymenocallidicola Crous, Summerell & Romberg, sp. nov. Phyllostictae citricarpae similis, sed conidiis minoribus, (8–)9–10(–11) × Typus. AUSTRALIA, Northern Territory, Darwin, Charles Darwin University, (6–)6.5–7 µm, discernitur. S 12°22'25.2" E 130°52'07.4" on leaves of Hymenocallis littoralis (Amaryl lidaceae), 1 May 2011, P.W. Crous & M. Romberg, holotype CBS H-20759, Etymology. Named after the host genus from which it was isolated, cultures ex-type CPC 19332, 19331 = CBS 131309, ITS sequence GenBank Hymenocallis. JQ044423 and LSU sequence GenBank JQ044443, MycoBank MB560699; Associated with brown leaf spots and leaf tip blight. Conidio Darwin, in front of Vibe Hotel, Kitchener Drive, Darwin Conference Centre, on leaves of Hymenocallis littoralis, 27 Apr. 2011, P.W. Crous & B.A. Summerell, mata pycnidial, solitary, black, erumpent, globose, exuding CBS H-20760, cultures CPC 19330, 19329 = CBS 131310, ITS sequence colourless to opaque conidial masses; pycnidia up to 200 µm GenBank JQ044424. diam; pycnidial wall of several layers of brown textura angula ris, up to 30 µm thick; inner layer of hyaline textura angularis. Notes — During a meeting of the Australasian Society for Ostiole central, up to 20 µm diam, rim lined with darker brown Plant Pathology in Darwin (April 2011), a serious leaf spot and cells. Conidiophores subcylindrical to ampulliform, reduced tip blight disease was noticed on the Hymenocallis littoralis to conidiogenous cells, or with 1–2 supporting cells, at times plants growing in front of the conference centre. -

Phylogeny and Biogeography of Hawkmoths (Lepidoptera: Sphingidae): Evidence from Five Nuclear Genes
Phylogeny and Biogeography of Hawkmoths (Lepidoptera: Sphingidae): Evidence from Five Nuclear Genes Akito Y. Kawahara1*, Andre A. Mignault1, Jerome C. Regier2, Ian J. Kitching3, Charles Mitter1 1 Department of Entomology, College Park, Maryland, United States of America, 2 Center for Biosystems Research, University of Maryland Biotechnology Institute, College Park, Maryland, United States of America, 3 Department of Entomology, The Natural History Museum, London, United Kingdom Abstract Background: The 1400 species of hawkmoths (Lepidoptera: Sphingidae) comprise one of most conspicuous and well- studied groups of insects, and provide model systems for diverse biological disciplines. However, a robust phylogenetic framework for the family is currently lacking. Morphology is unable to confidently determine relationships among most groups. As a major step toward understanding relationships of this model group, we have undertaken the first large-scale molecular phylogenetic analysis of hawkmoths representing all subfamilies, tribes and subtribes. Methodology/Principal Findings: The data set consisted of 131 sphingid species and 6793 bp of sequence from five protein-coding nuclear genes. Maximum likelihood and parsimony analyses provided strong support for more than two- thirds of all nodes, including strong signal for or against nearly all of the fifteen current subfamily, tribal and sub-tribal groupings. Monophyly was strongly supported for some of these, including Macroglossinae, Sphinginae, Acherontiini, Ambulycini, Philampelini, Choerocampina, and Hemarina. Other groupings proved para- or polyphyletic, and will need significant redefinition; these include Smerinthinae, Smerinthini, Sphingini, Sphingulini, Dilophonotini, Dilophonotina, Macroglossini, and Macroglossina. The basal divergence, strongly supported, is between Macroglossinae and Smerinthinae+Sphinginae. All genes contribute significantly to the signal from the combined data set, and there is little conflict between genes. -
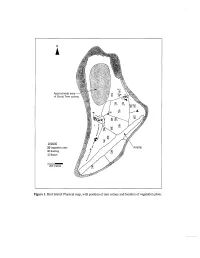
Figure 1. Bird Island: Physical Map, with Position of Tern Colony and Location of Vegetation Plots
Approximate of Sooty Ter~ Leqend (XI Vegetat~onplots 9 Building 6 Beach 200 metres Figure 1. Bird Island: Physical map, with position of tern colony and location of vegetation plots. BIRD MICHAEL J. HILL', TERENCE M. VEL', KATHRYN J. HOLM^, STEVEN J. PARR~ and NIRMAL J. SHAH' GEOLOGY, TOPOGRAPHY AND CLIMATE Bird is the northernmost island of the Seychelles, lying around 90 km north of Mahe, the largest of the granitic Seychelles, at the northern edge of the Seychelles bank. Different published sources vary in the estimated area of Bird Island with figures of c. 70 ha given by Feare (1979), 82 ha in Stoddart and Fosberg (1981), 101 ha in Skerrett et al. (2001), and 120.7 ha from recent aerial photographs (Ministry of Land Use and Habitat, Seychelles, unpublished data). In part, this variation may be explained by seasonal or longer-term variations in the vegetated area of the island; Bird Island is relatively dynamic, experiencing considerable coastal changes over time (Feare, 1979). The maximum elevation is less than 4 m above sea level. Unlike the majority of islands on the Seychelles Bank, Bird has no exposed granite and it is entirely formed of reef-derived sands. The accumulation of guano on sand deposits has led to the formation of phosphatic sandstone over 26% of the island's surface (Baker, 1963). Phosphatic sandstone is concentrated in a central band; the island's coastal zone is entirely sandy. Most of the original guano has now been removed for export. The soils of Bird Island are of two main series; over the central phosphatic sandstone area, Jemo series soils (missing their upper layer of guano) occur. -

Endemic Species of Christmas Island, Indian Ocean D.J
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 34 055–114 (2019) DOI: 10.18195/issn.0312-3162.34(2).2019.055-114 Endemic species of Christmas Island, Indian Ocean D.J. James1, P.T. Green2, W.F. Humphreys3,4 and J.C.Z. Woinarski5 1 73 Pozieres Ave, Milperra, New South Wales 2214, Australia. 2 Department of Ecology, Environment and Evolution, La Trobe University, Melbourne, Victoria 3083, Australia. 3 Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia. 4 School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, Western Australia 6009, Australia. 5 NESP Threatened Species Recovery Hub, Charles Darwin University, Casuarina, Northern Territory 0909, Australia, Corresponding author: [email protected] ABSTRACT – Many oceanic islands have high levels of endemism, but also high rates of extinction, such that island species constitute a markedly disproportionate share of the world’s extinctions. One important foundation for the conservation of biodiversity on islands is an inventory of endemic species. In the absence of a comprehensive inventory, conservation effort often defaults to a focus on the better-known and more conspicuous species (typically mammals and birds). Although this component of island biota often needs such conservation attention, such focus may mean that less conspicuous endemic species (especially invertebrates) are neglected and suffer high rates of loss. In this paper, we review the available literature and online resources to compile a list of endemic species that is as comprehensive as possible for the 137 km2 oceanic Christmas Island, an Australian territory in the north-eastern Indian Ocean. -

Phelsuma 22.Indd
Phelsuma 22 (2014); 1-5 Occurrence of the viable population of Chasmina candida (Walker, 1865) (Lepidoptera: Noctuidae: Bagisarinae) on Praslin Island, Seychelles Ivan N. Bolotov*, Vitaly M. Spitsin, Yulia S. Kolosova & Artem A. Frolov Institute of Ecological Problems of the North, Ural Branch of the Russian Academy of Sciences, Northern Dvina Emb., 23, 163000 Arkhangelsk, RUSSIAN FEDERATION *corresponding author: [email protected] Introduction The “large white” group of noctuids, belonging to the genus Chasmina Walker, 1856, includes at least fifteen virtually pure white species that are difficult to separate (Holloway 1989; Barnett et al. 1999). Chasmina candida (Walker, 1865) is a moderately rare species that is widely distributed in the Indo-Australian tropics to the east of Fiji, including many islands of the Indian Ocean and Pacific (Robinson 1974; Holloway 1989; Barnett et al. 1999). The northernmost species records are known from southern Japan (Kishida 2006). Usually, this species is observed in coastal areas, therefore, a recently published record from the mainland India may be erroneous (Gurule & Nikam 2013). Only Hibiscus tiliaceus L. and Thespecia populnea (L.) Sol. ex Corrêa (Malvaceae) are listed as valuable host plants for C. candida larvae (Gerlach & Matyot 2006; Leong 2010; Robinson et al. 2010). Both of these plant species are inhabitants of coastal ecosystems of mainland and oceanic islands, including beach crests and mangroves. Some authors noted that C. candida is associated with mangroves (Veenakumari & Prashanth 2009; Leong 2010). But, on the Fiji islands, the species is found in areas of secondary vegetation (Robinson 1974). Leong (2010) provided a description of the last instar larva and data on the species biology in Singapore. -

Sweetpotato Major Pests.P65
Sweetpotato:Sweetpotato:Sweetpotato: MajorMajorMajor PPPests,ests,ests, Diseases,Diseases, andand NutritionalNutritional DisordersDisordersDisorders T. Ames, N.E.J.M. Smit, A.R. Braun, J.N. O’Sullivan, and L.G. Skoglund ISBN 92-9060-187-6 Sweetpotato: Major Pests, Diseases, and Nutritional Disorders T. Ames, N.E.J.M. Smit, A.R. Braun, J.N. O’Sullivan, and L.G. Skoglund International Potato Center (CIP) C O N T E N T S The International Potato Center (CIP) is a scientific, nonprofit institution dedicated to the increased and more sustainable use of potato, Page sweetpotato, and other roots and tubers in the Foreword vii developing world, and to the improved management of agricultural resources in the Acknowledgments viii Andes and other mountain areas. CIP is part of the global agricultural research network known as the Consultative Group on Introduction 1 International Agricultural Research (CGIAR). CGIAR Insect Pests of Sweetpotato and Their Management 3 International Potato Center Apartado 1558 Storage Root Feeders 4 Lima 12, Peru Sweetpotato Weevils (Cylas spp.) 4 West Indian Sweetpotato Weevil (Euscepes ISBN 92-9060-187-6 postfasciatus)10 Press run: 1000 Rough Sweetpotato Weevil (Blosyrus sp.) 12 Printed in Lima, Peru August, 1997 Clearwing Moth (Synanthedon spp.) 14 Peloropus Weevil (Peloropus batatae)14 Cover: Photo of chlorotic spots with and without purple margins induced White Grubs 15 by SPFMV (taken by S. Fuentes). Stemborers and Feeders 16 T. Ames, N.E.J.M. Smit, A.R. Braun, J.N. O’Sullivan, and L.G. Skoglund. Clearwing Moth (Synanthedon spp.) 16 1996. Sweetpotato: Major Pests, Diseases, and Nutritional Disorders. -

The Lepidopteran Pests of Sweet Potato
Türk. entomol. derg., 2016, 40 (2): 149-156 ISSN 1010-6960 DOI: http://dx.doi.org/10.16970/ted.81457 Original article (Orijinal araştırma) The lepidopteran pests of sweet potato: First record of Helcystogramma triannulella (Herrich-Schäffer) (Lepidoptera: Gelechiidae) with population development and natural enemies in Turkey1 Tatlı patatesteki zararlı lepidopterler: Türkiye’de Helcystogramma triannulella (Herrich-Schäffer) (Lepidoptera: Gelechiidae)’nın ilk kaydı, popülasyon gelişmesi ve doğal düşmanları Kamuran KAYA2* Feza CAN CENGIZ3 Mehmet Emin ÇALIŞKAN4 Sevgi ÇALIŞKAN4 Summary The study was conducted in Hatay Province, east Mediterranean Region, Turkey in 2012 and 2013 to determine major lepidopteran pests of sweet potato and to document the population development of the newly recorded species Helcystogramma triannulella (Herrich-Schäffer) (Lepidoptera: Gelechiidae) in 2013. Four lepidopteran species, Aedia leucomelas (L.) (Noctuidae), Agrius convolvuli (Linnaeus) (Sphingidae), Hydriris ornatalis (Duponchel) (Crambidae) and H. triannulella were found. This was the first detection of H. triannulella in Turkey, so its population development was studied in the second year. The larval population of H. triannulella began to increase towards the end of July and reached its peak in mid-August. During the study, predators, Hippodamia variegata (Goeze), Oenopia conglobata (L.), Scymnus interruptus (Goeze), Scymnus mediterraneus Khnzorian, Stethorus gilvifrons (Mulsant) (Coleoptera; Coccinellidae); Nabis viridulus Spinola (Hemiptera: Nabidae)