Static Electricity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Printer Tech Tips—Cause & Effects of Static Electricity in Paper
Printer Tech Tips Cause & Effects of Static Electricity in Paper Problem The paper has developed a static electrical charge causing an abnormal sheet-to- sheet or sheet-to-material attraction which is difficult to separate. This condition may result in feeder trip-offs, print voids from surface contamination, ink offset, Sappi Printer Technical Service or poor sheet jog in the delivery. 877 SappiHelp (727 7443) Description Static electricity is defined as a non-moving, non-flowing electrical charge or in simple terms, electricity at rest. Static electricity becomes visible and dynamic during the brief moment it sparks a discharge and for that instant it’s no longer at rest. Lightning is the result of static discharge as is the shock you receive just before contacting a grounded object during unusually dry weather. Matter is composed of atoms, which in turn are composed of protons, neutrons, and electrons. The number of protons and neutrons, which make up the atoms nucleus, determine the type of material. Electrons orbit the nucleus and balance the electrical charge of the protons. When both negative and positive are equal, the charge of the balanced atom is neutral. If electrons are removed or added to this configuration, the overall charge becomes either negative or positive resulting in an unbalanced atom. Materials with high conductivity, such as steel, are called conductors and maintain neutrality because their electrons can move freely from atom to atom to balance any applied charges. Therefore, conductors can dissipate static when properly grounded. Non-conductive materials, or insulators such as plastic and wood, have the opposite property as their electrons can not move freely to maintain balance. -

Teaching H. C. Ørsted's Scientific Work in Danish High School Physics
UNIVERSITY OF COPENHAGEN FACULTY OF SCIENCE Ida Marie Monberg Hindsholm Teaching H. C. Ørsted's Scientific Work in Danish High School Physics Masterʹs thesis Department of Science Education 19 July 2018 Master's thesis Teaching H. C. Ørsted’s Scientific Work in Danish High School Physics Submitted 19 July 2018 Author Ida Marie Monberg Hindsholm, B.Sc. E-mail [email protected] Departments Niels Bohr Institute, University of Copenhagen Department of Science Education, University of Copenhagen Main supervisor Ricardo Avelar Sotomaior Karam, Associate Professor, Department of Science Education, University of Copenhagen Co-supervisor Steen Harle Hansen, Associate Professor, Niels Bohr Institute, University of Copenhagen 1 Contents 1 Introduction . 1 2 The Material: H. C. Ørsted's Work . 3 2.1 The Life of Hans Christian Ørsted . 3 2.2 Ørsted’s Metaphysical Framework: The Dynamical Sys- tem............................. 6 2.3 Ritter and the failure in Paris . 9 2.4 Ørsted’s work with acoustic and electric figures . 12 2.5 The discovery of electromagnetism . 16 2.6 What I Use for the Teaching Sequence . 19 3 Didactic Theory . 20 3.1 Constructivist teaching . 20 3.2 Inquiry Teaching . 22 3.3 HIPST . 24 4 The Purpose and Design of the Teaching Sequence . 27 4.1 Factual details and lesson plan . 28 5 Analysis of Transcripts and Writings . 40 5.1 Method of Analysis . 40 5.2 Practical Problems . 41 5.3 Reading Original Ørsted's Texts . 42 5.4 Inquiry and Experiments . 43 5.5 "Role play" - Thinking like Ørsted . 48 5.6 The Reflection Corner . 51 5.7 Evaluation: The Learning Objectives . -

Materials That Cause Static Electricity
Materials that Cause Static Electricity http://www.school-for-champions.com/science/static_materials.htm Some materials cause or create more static electricity than others. Since static electricity is the collection of electrically charged particles on the surface of a material, various materials have a tendency of either giving up electrons and becoming positive (+) in charge or attracting electrons and becoming negative (−) in charge. The Triboelectric Series is a list of materials, showing which have a greater tendency to become positive (+) and which have a greater tendency to become negative (−). The list is a handy tool to determine which combinations of materials create the most static electricity. Questions you may have include: What are materials in the Triboelectric Series? What are the best combinations of materials? What are acceptable combinations? Triboelectric Series Common materials are listed according how well they create static electricity when rubbed with another material, as well as what charge the material will possess. Become positive in charge The following materials will tend to give up electrons when brought in contact with other materials. They are listed from those with the greatest tendency to give electrons to those that barely give up electrons. Materials that gain a positive (+) electrical charge (or tend to give up electrons) Greatest tendency to giving up electrons and becoming highly positive (+) in Dry human skin charge Leather Rabbit fur Fur is often used to create static electricity Glass The glass on your TV screen gets charged and collects dust Human hair "Flyaway hair" is a good example of having a moderate positive (+) charge Nylon Wool Lead A surprise that lead would collect as much static electricity as cat fur Cat fur Silk Aluminum Gives up some electrons Paper Neutral There are very few materials that do not tend to readily attract or give up electrons when brought in contact or rubbed with other materials. -

When You Pull a Wool Sweater Over Your Head, You Might Look Like This
NAME__________________ 6. Who discovered that lightning was a form of Science SOL 4.3 – Electricity electricity? www.solpass.org a. Thomas Edison b. Benjamin Franklin When you pull a wool sweater quickly c. Michael Faraday over your head, you might look like this. When Electric current powers many things in our homes. your feet rub the carpet before you touch a metal Current electricity is the flow of electrons through a doorknob you might get a shock or see a spark. conductor. Most home appliances need current electricity Why? Static electricity. Static electricity is the running through an electrical circuit in order to work. An open circuit is a circuit with a break in it that will result of electrons being pulled from not allow the flow of electrons. A closed circuit allows the one surface to another when two objects flow of electrons. rub together. This can happen if you take off a sweater too fast or when you 7. A(n) ______ will allow the flow of electricity. scuff your feet on the carpet. Your feet pick up extra electrons a. open circuit from the carpet and become negatively charged. Those electrons jump off your finger as a spark when your finger comes close to a b. closed circuit metal object like a doorknob. 8. The diagram shows a(n) 1. Which of the following creates static electricity? a. closed a. Taking a bath circuit b. Plugging a power cord into the wall outlet. b. open c. Rubbing feet on the carpet. circuit 2. What might cause foam 9. -

Chapter 7 Electricity Lesson 2 What Are Static and Current Electricity?
Chapter 7 Electricity Lesson 2 What Are Static and Current Electricity? Static Electricity • Most objects have no charge= the atoms are neutral. • They have equal numbers of protons and electrons. • When objects rub against another, electrons move from the atoms of one to atoms of the other object. • The numbers of protons and electrons in the atoms are no longer equal: they are either positively or negatively charged. • The buildup of charges on an object is called static electricity. • Opposite charges attract each other. • Charged objects can also attract neutral objects. • When items of clothing rub together in a dryer, they can pick up a static charge. • Because some items are positive and some are negative, they stick together. • When objects with opposite charges get close, electrons sometimes jump from the negative object to the positive object. • This evens out the charges, and the objects become neutral. • The shocks you can feel are called static discharge. • The crackling noises you hear are the sounds of the sparks. • Lightning is also a static discharge. • Where does the charge come from? • Scientists HYPOTHESIZE that collisions between water droplets in a cloud cause the drops to become charged. • Negative charges collect at the bottom of the cloud. • Positive charges collect at the top of the cloud. • When electrons jump from one cloud to another, or from a cloud to the ground, you see lightning. • The lightning heats the air, causing it to expand. • As cooler air rushes in to fill the empty space, you hear thunder. • Earth can absorb lightning’s powerful stream of electrons without being damaged. -
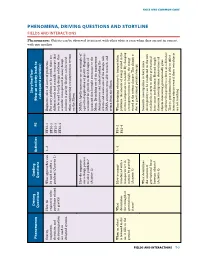
Phenomena, Driving Questions and Storyline Fields and Interactions
with oneanother. Phenomenon: FIELDS AND INTERACTIONS AND FIELDS PHENOMENA, DRIVING QUESTIONS AND STORYLINE AND QUESTIONS DRIVING PHENOMENA, Storyline/Flow Driving Guiding Phenomenon Activities PE (How an activity leads to Questions Questions subsequent activities) Gravity, How do What approaches can 1, 2 ETS1-1 Engineers solve all sorts of problems. Objectscanbeobserved whentheyarenotincontact tointeractwithotherobjectseven magnetism, engineers solve be used to solve a ETS1-2 For every problem to be solved, there are electricity, and problems related problem? (Activity 1) ETS1-3 different tools and scientifc concepts that electromagnetism to gravity? can be used to help design solutions. One ETS1-4 are used in scientifc concept used in many design designed systems. solutions is gravity. Gravity can be used when engineering a transportation system on the Moon. How do engineers NASA’s Apollo missions are an example of use a design process a practical application of gravity. Engineers to solve problems? accounted for gravity in their designs of (Activity 2) spacecraft that brought astronauts to the Moon. By making use of the engineering design process and understanding the criteria and constraints of the design task, NASA astronauts were able to reach, and return from, the Moon. When an object What How is energy 3, 4 PS3-2 When designing solutions to transportation is released in the determines transferred with a PS2-4 problems, the amount of energy stored in the air, it falls to the the strength of transporter set in system is important to consider. By changing ground. gravitational motion by gravity? a transporter’s mass or height, the energy FIELDS FIELDS AND INTERACTIONS forces? (Activity 3) stored in the system changes. -

H. C. Ørsted and the Discovery of Electromagnetism During a Lecture Given in the Spring of 1820 Hans Christian Ørsted De- Cided to Perform an Experiment
H. C. Ørsted and the Discovery of Electromagnetism During a lecture given in the spring of 1820 Hans Christian Ørsted de- cided to perform an experiment. As he described it years later, \The plan of the first experiment was, to make the current of a little galvanic trough apparatus, commonly used in his lectures, pass through a very thin platina wire, which was placed over a compass covered with glass. The preparations for the experiments were made, but some accident having hindered him from trying it before the lec- ture, he intended to defer it to another opportunity; yet during the lecture, the probability of its success appeared stronger, so that he made the first experiment in the presence of the audience. The mag- netical needle, though included in a box, was disturbed; but as the effect was very feeble, and must, before its law was discovered, seem very irregular, the experiment made no strong impression on the au- dience. It may appear strange, that the discoverer made no further experiments upon the subject during three months; he himself finds it difficult enough to conceive it; but the extreme feebleness and seeming confusion of the phenomena in the first experiment, the remembrance of the numerous errors committed upon this subject by earlier philoso- phers, and particularly by his friend Ritter, the claim such a matter has to be treated with earnest attention, may have determined him to delay his researches to a more convenient time. In the month of July 1820, he again resumed the experiment, making use of a much more considerable galvanical apparatus."1 The effect that Ørsted observed may have been \very feeble". -

Electricity and Magnetism
ELECTRICITY AND MAGNETISM UNIT OVERVIEW We use electricity and magnetism every day, but how do they each work? How are they related? The Electricity and Magnetism unit explains electricity, from charged particles at the atomic level to the current that flows in homes and businesses. There are two kinds of electricity: static electricity and electric currents. There are also two kinds of electric currents: direct (DC) and alternating (AC). Electricity and magnetism are closely related. Flowing electrons produce a magnetic field, and spinning magnets cause an electric current to flow. Electromagnetism is the interaction of these two important forces. Electricity and magnetism are integral to the workings of nearly every gadget, appliance, vehicle, and machine we use. Certain reading resources are provided at three reading levels within the unit to support differentiated instruction. Other resources are provided as a set, with different titles offered at each reading level. Dots on student resources indicate the reading level as follows: low reading level middle reading level high reading level THE BIG IDEA Without electricity, we’d literally be in the dark. We’d be living in a world lit by open flame and powered by simple machines that rely on muscle power. Since the late 1800s, electricity has brightened our homes and streets, powered our appliances, and enabled the development of computers, phones, and many other devices we rely on. But people often take electricity for granted. Flip a switch and it’s there. Understanding what electricity is and how it becomes ready for our safe use helps us appreciate this energy source. -

History of Magnetism and Electricity History of Magnetism and Electricity
History of Magnetism and Electricity History of Magnetism and Electricity ● As the result of successfully completing this unit, the students will – Discuss the historical background of electricity, electromagnetism, and circuits – Compare and Contrast the time frame needed to discover the basic laws of electromagnetism and the time frame this course is taking to introduce those same concepts to the students Static Electricity – Thales from Milet ● Ca 600 BC ● Amber rubbed will attract light objects sources: http://en.wikipedia.org/wiki/File:Thales.jpg Static Electricity Static Electricity Static Electricity Static Electricity Static Electricity Static Electricity Static Electricity Static Electricity – Thales from Milet ● Ca 600 BC ● Amber rubbed will attract light objects sources: http://en.wikipedia.org/wiki/File:Thales.jpg Static Electricity – Thales from Milet ● Ca 600 BC ● Amber rubbed will attract light objects ● ηλεκτρον (greek for amber) sources: http://en.wikipedia.org/wiki/File:Thales.jpg Static Electricity η λ ε κ τ ρ ο ν η = Eta λ = Lambda ε = Epsilon κ = Kappa τ = Tau ρ = Rho ο = Omega ν = Nu Static Electricity η λ ε κ τ ρ ο ν η = E E L E K T R O N λ = L ε = E κ = K τ = T ρ = R ο = O ν = N Static Electricity – Thales from Milet ● Ca 600 BC ● Amber rubbed will attract light objects ● ηλεκτρον (greek for amber) → electron sources: http://en.wikipedia.org/wiki/File:Thales.jpg William Gilbert - Magnetism ● 1600 sources: http://en.wikipedia.org/wiki/File:William_Gilbert.jpg http://www.solarnavigator.net/compass.htm http://www.physics.ubc.ca/~outreach/phys420/p420_01/shaun/shaun/why_it_works.htm -

Electricity and Magnetism
Electricity and Magnetism Definition The Physical phenomena involving electric charges, their motions, and their effects. The motion of a charge is affected by its interaction with the electric field and, for a moving charge, the magnetic field. The electric field acting on a charge arises from the presence of other charges and from a time-varying magnetic field. The magnetic field acting on a moving charge arises from the motion of other charges and from a time-varying electric field. Thus electricity and magnetism are ultimately inextricably linked. In many cases, however, one aspect may dominate, and the separation is meaningful. See also Electric charge; Electric field; Magnetism. History of Electricity From the writings of Thales of Miletus it appears that Westerners knew as long ago as 600 B.C. that amber becomes charged by rubbing. There was little real progress until the English scientist William Gilbert in 1600 described the electrification of many substances and coined the term electricity from the Greek word for amber. As a result, Gilbert is called the father of modern electricity. In 1660 Otto von Guericke invented a crude machine for producing static electricity. It was a ball of sulfur, rotated by a crank with one hand and rubbed with the other. Successors, such as Francis Hauksbee, made improvements that provided experimenters with a ready source of static electricity. Today's highly developed descendant of these early machines is the Van de Graaf generator, which is sometimes used as a particle accelerator. Robert Boyle realized that attraction and repulsion were mutual and that electric force was transmitted through a vacuum (c.1675). -

What Is the Difference? Anti-Static, ESD, Conductive, Insulative?
FAQ Information What is the Difference Between Anti-Static, Dissipative, Conductive, and Insulative? Static Electricity As the name implies, static electricity is electricity at rest. The electrical charge is the transference of electrons that occurs when there is sliding, rubbing, or separating of a material, which is a generator of electrostatic voltages. For example: plastics, fiber glass, rubber, textiles, etc. Under the right conditions, this induced charge can reach 30,000 to 40,000 volts. When this happens to an insulating material, like plastic, the charge tends to remain in the localized area of contact. This electrostatic voltage may then discharge via an arc or spark when the plastic material comes in contact with a body at a sufficiently different potential, such as a person or microcircuit. If Electrostatic Discharge (ESD) occurs to a person, the results may range anywhere from a mild to a painful shock. Extreme cases of ESD, or Arc Flash, can even result in loss of life. These types of sparks are especially dangerous in environments that may contain flammable liquids, solids or gasses, such as a hospital operating room or explosive device assembly. Some micro-electronic parts can be destroyed or damaged by ESD as low as 20 volts. Since people are prime causes of ESD, they often cause damage to sensitive electronic parts, especially during manufacturing and assembly. The consequences of discharge through an electrical component sensitive to ESD can range from erroneous readings to permanent damage resulting in excessive equipment downtime and costly repair or total part replacement. Electrostatic Discharge (ESD) The sudden flow of electricity between two electrically charged objects caused by contact, an electrical short, or dielectric breakdown. -

7. Static Electricity and Capacitance
Leaving Cert Physics Long Questions 2018 - 2002 7. Static Electricity and Capacitance Please remember to photocopy 4 pages onto one sheet by going A3→A4 and using back to back on the photocopier Contents Static electricity: ordinary level questions ........................................................................................................................ 2 Static electricity: higher level questions ........................................................................................................................... 3 Electric fields / electric field strength (all higher level) .................................................................................................... 4 Capacitance: ordinary level questions .............................................................................................................................. 7 Capacitance: higher level questions ................................................................................................................................. 9 Solutions to higher level questions ................................................................................................................................. 11 1 Static electricity: ordinary level questions 2013 Question 12 (c) [Ordinary Level] (i) State Coulomb’s law of force between electric charges. (ii) The diagram shows a positively-charged electroscope. Give a use for an electroscope. (iii)How can an electroscope be given a positive charge? (iv) What is observed if you touch the cap of the electroscope