Phylogenomic Study of Burkholderia Glathei-Like Organisms, Proposal Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multilayered Horizontal Operon Transfers from Bacteria Reconstruct a Thiamine Salvage Pathway in Yeasts
Multilayered horizontal operon transfers from bacteria reconstruct a thiamine salvage pathway in yeasts Carla Gonçalvesa and Paula Gonçalvesa,1 aApplied Molecular Biosciences Unit-UCIBIO, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal Edited by Edward F. DeLong, University of Hawaii at Manoa, Honolulu, HI, and approved September 22, 2019 (received for review June 14, 2019) Horizontal acquisition of bacterial genes is presently recognized as nisms presumed to have facilitated a transition from bacterial an important contribution to the adaptation and evolution of operon transcription to eukaryotic-style gene expression were eukaryotic genomes. However, the mechanisms underlying ex- proposed, such as gene fusion giving rise to multifunctional pro- pression and consequent selection and fixation of the prokaryotic teins (6, 23, 24), increase in intergenic distances between genes to genes in the new eukaryotic setting are largely unknown. Here we generate room for eukaryotic promoters, and independent tran- show that genes composing the pathway for the synthesis of the scription producing mRNAs with poly(A) tails have been dem- essential vitamin B1 (thiamine) were lost in an ancestor of a yeast onstrated (22). In the best documented study, which concerns a lineage, the Wickerhamiella/Starmerella (W/S) clade, known to bacterial siderophore biosynthesis operon acquired by yeasts be- harbor an unusually large number of genes of alien origin. The longing to the Wickerhamiella/Starmerella (W/S) clade, the bacte- thiamine pathway was subsequently reassembled, at least twice, rial genes acquired as an operon were shown to be functional (22). by multiple HGT events from different bacterial donors involving Thiamine, commonly known as vitamin B1, is essential for all both single genes and entire operons. -

The Burkholderia Genus: Between Mutualism and Pathogenicity
The Burkholderia genus: between mutualism and pathogenicity El género Burkholderia: entre el mutualismo y la patogenicidad David Espinosa-Victoria*, Laboratorio Interacción Molecular Planta-Microorganismo, 1Programa de Edafo- logía Colegio de Postgraduados, Carretera México-Texcoco Km 36.5, Montecillo Estado de México, México, 56230; Lucía López-Reyes, Moisés Graciano Carcaño-Montiel, Laboratorio Microbiología de Suelos, Bene- mérita Universidad Autónoma de Puebla, Avenida San Claudio s/n, Ciudad Universitaria, La Hacienda, Puebla, Puebla, 72592; 1María Serret-López. *Autor para correspondencia: [email protected] Recibido: 28 de Abril, 2020. Aceptado: 04 de Junio, 2020. Espinosa-Victoria D, López-Reyes L, Carcaño-Montiel Abstract. Burkholderia is an ambivalent genus MG and Serret-López M. 2020. The Burkholderia ge- because some of its species establish symbiotic- nus: between mutualism and pathogenicity. Mexican Jo- mutualistic relationships with plants, and urnal of Phytopathology 38(3): 337-359. symbiotic-pathogenic relationships with plants, DOI: 10.18781/R.MEX.FIT.2004-5 animals, and humans. Since the phytopathogenic bacterium B. cepacia was reported as a nosocomial Primera publicación DOI: 17 de Junio, 2020. opportunist, associated with cystic fibrosis, the First DOI publication: June 17, 2020. concern about possible infections in humans arose. The objective of this contribution was to make an analysis of Burkholderia’s functional versatility Resumen. Burkholderia es un género ambivalen- and its effect on human health. Burkholderia te debido a que algunas de sus especies establecen harbored about 100 species and the B. cepacia relaciones simbiótico-mutualistas con las plantas, y complex (BCC) consisting of 22 species. At the simbiótico-patogénicas con plantas, animales y hu- beginning, the existence of two lineages within manos. -

Burkholderia Cenocepacia Integrates Cis-2-Dodecenoic Acid and Cyclic Dimeric Guanosine Monophosphate Signals to Control Virulence
Burkholderia cenocepacia integrates cis-2-dodecenoic acid and cyclic dimeric guanosine monophosphate signals to control virulence Chunxi Yanga,b,c,d,1, Chaoyu Cuia,b,c,1, Qiumian Yea,b, Jinhong Kane, Shuna Fua,b, Shihao Songa,b, Yutong Huanga,b, Fei Hec, Lian-Hui Zhanga,c, Yantao Jiaf, Yong-Gui Gaod, Caroline S. Harwoodb,g,2, and Yinyue Denga,b,c,2 aState Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University, Guangzhou 510642, China; bGuangdong Innovative Research Team of Sociomicrobiology, College of Agriculture, South China Agricultural University, Guangzhou 510642, China; cIntegrative Microbiology Research Centre, South China Agricultural University, Guangzhou 510642, China; dSchool of Biological Sciences, Nanyang Technological University, Singapore 637551; eCenter for Crop Germplasm Resources, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China; fState Key Laboratory of Plant Genomics, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China; and gDepartment of Microbiology, University of Washington, Seattle, WA 98195 Contributed by Caroline S. Harwood, October 30, 2017 (sent for review June 1, 2017; reviewed by Maxwell J. Dow and Tim Tolker-Nielsen) Quorum sensing (QS) signals are used by bacteria to regulate N-octanoyl homoserine lactone (C8-HSL). The two QS systems biological functions in response to cell population densities. Cyclic have both distinct and overlapping effects on gene expression diguanosine monophosphate (c-di-GMP) regulates cell functions in (16, 17). response to diverse environmental chemical and physical signals One of the ways in which QS systems can act is by controlling that bacteria perceive. In Burkholderia cenocepacia, the QS signal levels of intracellular cyclic diguanosine monophosphate (c-di-GMP) receptor RpfR degrades intracellular c-di-GMP when it senses the in bacteria (18–21). -

Short-Interval Reburns in the Boreal Forest Alter Soil Bacterial Communities, Reflecting Increased Ph and Poor Conifer Seedling
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.31.437944; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Short-interval reburns in the boreal forest alter soil bacterial communities, reflecting 2 increased pH and poor conifer seedling establishment 3 4 Jamie Wooleta,b, Ellen Whitmanc,d, Marc-André Parisienc, Dan K. Thompsonc, Mike D. 5 Flannigand, and Thea Whitmana* 6 a. Department of Soil Science, University of Wisconsin-Madison, 1525 Observatory Dr., 7 Madison, WI, 53706, USA 8 b. Department of Forest and Rangeland Stewardship, Colorado State University, 1001 Amy Van 9 Dyken Way, Fort Collins, CO, 80521, USA 10 c. Northern Forestry Centre, Canadian Forest Service, Natural Resources Canada, 5320 122 11 Street, Edmonton, AB, T6H 3S5, Canada 12 d. Department of Renewable Resources, University of Alberta, 751 General Services Building, 13 Edmonton, AB, T6G 2H1, Canada 14 * Corresponding author: [email protected]; 608.263.4947 15 16 Abstract: Increasing burn rates (percentage area burned annually) in some biomes are leading to 17 fires burning in close succession, triggering rapid vegetation change as well as altering soil 18 properties. Despite the importance of soil microbes for nutrient cycling and as plant symbionts, 19 the effects of increased fire frequency on belowground microbial communities remain largely 20 unknown. We present a study of the effects of short interval reburns (defined here as <20 years 21 between fires) on soil bacterial communities in the boreal forest of northwestern Canada, using a 22 paired site design that spans wetlands and uplands, with 50 sites total. -

Burkholderia Cenocepacia Intracellular Activation of the Pyrin
Activation of the Pyrin Inflammasome by Intracellular Burkholderia cenocepacia Mikhail A. Gavrilin, Dalia H. A. Abdelaziz, Mahmoud Mostafa, Basant A. Abdulrahman, Jaykumar Grandhi, This information is current as Anwari Akhter, Arwa Abu Khweek, Daniel F. Aubert, of September 29, 2021. Miguel A. Valvano, Mark D. Wewers and Amal O. Amer J Immunol 2012; 188:3469-3477; Prepublished online 24 February 2012; doi: 10.4049/jimmunol.1102272 Downloaded from http://www.jimmunol.org/content/188/7/3469 Supplementary http://www.jimmunol.org/content/suppl/2012/02/24/jimmunol.110227 Material 2.DC1 http://www.jimmunol.org/ References This article cites 71 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/188/7/3469.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 29, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2012 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Activation of the Pyrin Inflammasome by Intracellular Burkholderia cenocepacia Mikhail A. -

Genomic Plasticity of the Causative Agent of Melioidosis, Burkholderia Pseudomallei
Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei Matthew T. G. Holdena, Richard W. Titballb,c, Sharon J. Peacockd,e, Ana M. Cerden˜ o-Ta´ rragaa, Timothy Atkinsb, Lisa C. Crossmana, Tyrone Pittf, Carol Churchera, Karen Mungalla, Stephen D. Bentleya, Mohammed Sebaihiaa, Nicholas R. Thomsona, Nathalie Basona, Ifor R. Beachamg, Karen Brooksa, Katherine A. Brownh, Nat F. Browng, Greg L. Challisi, Inna Cherevacha, Tracy Chillingwortha, Ann Cronina, Ben Crossetth, Paul Davisa, David DeShazerj, Theresa Feltwella, Audrey Frasera, Zahra Hancea, Heidi Hausera, Simon Holroyda, Kay Jagelsa, Karen E. Keithh, Mark Maddisona, Sharon Moulea, Claire Pricea, Michael A. Quaila, Ester Rabbinowitscha, Kim Rutherforda, Mandy Sandersa, Mark Simmondsa, Sirirurg Songsivilaik, Kim Stevensa, Sarinna Tumapae, Monkgol Vesaratchaveste, Sally Whiteheada, Corin Yeatsa, Bart G. Barrella, Petra C. F. Oystonb, and Julian Parkhilla,l aWellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SA, United Kingdom; bDefence Science and Technology Laboratory, Porton Down, Salisbury SP4 0JQ, United Kingdom; cDepartment of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London WC1E 7HT, United Kingdom; dNuffield Department of Clinical Medicine, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, United Kingdom; eFaculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand; fLaboratory of Hospital Infection, Division of Nosocomial Infection Prevention and Control, Central Public Health Laboratory, London NW9 5HT, United Kingdom; gSchool of Health Science, Griffith University, Gold Coast, Queensland 9726, Australia; hDepartment of Biological Sciences, Centre for Molecular Microbiology and Infection, Flowers Building, Imperial College, London SW7 2AZ, United Kingdom; iDepartment of Chemistry, University of Warwick, Coventry CV4 7AL, United Kingdom; jU.S. -

Characterization of the Burkholderia Cenocepacia J2315 Surface-Exposed Immunoproteome
Article Characterization of the Burkholderia cenocepacia J2315 Surface-Exposed Immunoproteome 1, 1, 1, Sílvia A. Sousa * , António M.M. Seixas y , Manoj Mandal y, Manuel J. Rodríguez-Ortega 2 and Jorge H. Leitão 1,* 1 iBB–Institute for Bioengineering and Biosciences, 1049-001 Lisbon, Portugal; [email protected] (A.M.M.S.); [email protected] (M.M.) 2 Departament of Biochemistry and Molecular Biology, Córdoba University, 14071 Córdoba, Spain; [email protected] * Correspondence: [email protected] (S.A.S.); [email protected] (J.H.L.); Tel.: +351-2184-19986 (S.A.S.); +351-2184-17688 (J.H.L.) Both authors contributed equally to the work. y Received: 31 July 2020; Accepted: 3 September 2020; Published: 6 September 2020 Abstract: Infections by the Burkholderia cepacia complex (Bcc) remain seriously life threatening to cystic fibrosis (CF) patients, and no effective eradication is available. A vaccine to protect patients against Bcc infections is a highly attractive therapeutic option, but none is available. A strategy combining the bioinformatics identification of putative surface-exposed proteins with an experimental approach encompassing the “shaving” of surface-exposed proteins with trypsin followed by peptide identification by liquid chromatography and mass spectrometry is here reported. The methodology allowed the bioinformatics identification of 263 potentially surface-exposed proteins, 16 of them also experimentally identified by the “shaving” approach. Of the proteins identified, 143 have a high probability of containing B-cell epitopes that are surface-exposed. The immunogenicity of three of these proteins was demonstrated using serum samples from Bcc-infected CF patients and Western blotting, validating the usefulness of this methodology in identifying potentially immunogenic surface-exposed proteins that might be used for the development of Bcc-protective vaccines. -

The Potato Yam Phyllosphere Ectosymbiont Paraburkholderia Sp
fmicb-11-00581 April 19, 2020 Time: 8:50 # 1 ORIGINAL RESEARCH published: 21 April 2020 doi: 10.3389/fmicb.2020.00581 The Potato Yam Phyllosphere Ectosymbiont Paraburkholderia sp. Msb3 Is a Potent Growth Promotor in Tomato Johannes B. Herpell1, Florian Schindler1, Mersad Bejtovic´ 1, Lena Fragner1,2, Bocar Diallo1, Anke Bellaire3, Susanne Kublik4, Bärbel U. Foesel4, Silvia Gschwendtner4, Melina Kerou5, Michael Schloter4 and Wolfram Weckwerth1,2* 1 Molecular Systems Biology (MOSYS), Department of Functional and Evolutionary Ecology, University of Vienna, Vienna, Austria, 2 Vienna Metabolomics Center (VIME), University of Vienna, Vienna, Austria, 3 Division of Structural and Functional Botany, Department of Botany and Biodiversity Research, University of Vienna, Vienna, Austria, 4 Research Unit Edited by: for Comparative Microbiome Analysis, German Research Center for Environmental Health, Helmholtz Zentrum München, 5 Michele Perazzolli, Neuherberg, Germany, Archaea Biology and Ecogenomics Division, Department of Functional and Evolutionary Ecology, University of Trento, Italy University of Vienna, Vienna, Austria Reviewed by: Stéphane Compant, The genus Paraburkholderia includes a variety of species with promising features for Austrian Institute of Technology (AIT), sustainable biotechnological solutions in agriculture through increasing crop productivity. Austria Lionel Moulin, Here, we present a novel Paraburkholderia isolate, a permanent and predominant Institut de Recherche Pour le member of the Dioscoreae bulbifera (yam family, Dioscoreaceae) -

Targeting the Burkholderia Cepacia Complex
viruses Review Advances in Phage Therapy: Targeting the Burkholderia cepacia Complex Philip Lauman and Jonathan J. Dennis * Department of Biological Sciences, University of Alberta, Edmonton, AB T6G 2E9, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-780-492-2529 Abstract: The increasing prevalence and worldwide distribution of multidrug-resistant bacterial pathogens is an imminent danger to public health and threatens virtually all aspects of modern medicine. Particularly concerning, yet insufficiently addressed, are the members of the Burkholderia cepacia complex (Bcc), a group of at least twenty opportunistic, hospital-transmitted, and notoriously drug-resistant species, which infect and cause morbidity in patients who are immunocompromised and those afflicted with chronic illnesses, including cystic fibrosis (CF) and chronic granulomatous disease (CGD). One potential solution to the antimicrobial resistance crisis is phage therapy—the use of phages for the treatment of bacterial infections. Although phage therapy has a long and somewhat checkered history, an impressive volume of modern research has been amassed in the past decades to show that when applied through specific, scientifically supported treatment strategies, phage therapy is highly efficacious and is a promising avenue against drug-resistant and difficult-to-treat pathogens, such as the Bcc. In this review, we discuss the clinical significance of the Bcc, the advantages of phage therapy, and the theoretical and clinical advancements made in phage therapy in general over the past decades, and apply these concepts specifically to the nascent, but growing and rapidly developing, field of Bcc phage therapy. Keywords: Burkholderia cepacia complex (Bcc); bacteria; pathogenesis; antibiotic resistance; bacterio- phages; phages; phage therapy; phage therapy treatment strategies; Bcc phage therapy Citation: Lauman, P.; Dennis, J.J. -

LIST of PROKARYOTIC NAMES VALIDLY PUBLISHED in August 2016
Leibniz‐Institut DSMZ‐Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH LIST OF PROKARYOTIC NAMES VALIDLY PUBLISHED in August 2016 compiled by Dorothea Gleim, Leibniz‐Institut DSMZ ‐ Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH Braunschweig, Germany PROKARYOTIC NOMENCLATURE, Update 08/2016 2 Notes This compilation of validly published names of Prokaryotes is produced to the best of our knowledge. Nevertheless we do not accept any responsibility for errors, inaccuracies or omissions. Names of prokaryotes are defined as being validly published by the International Code of Nomenclature of Bacteria a,b. Validly published are all names which are included in the Approved Lists of Bacterial Names c,d,e,f and the names subsequently published in the International Journal of Systematic Bacteriology (IJSB) and, from January 2000, in the International Journal of Systematic and Evolutionary Microbiology (IJSEM) in the form of original articles or in the Validation Lists. Names not considered to be validly published, should no longer be used, or used in quotation marks, i.e. “Streptococcus equisimilis” to denote that the name has no standing in nomenclature. Please note that such names cannot be retrieved in this list. Explanations, Examples Numerical reference followed by Streptomyces setonii 30:401 (AL) Included in The Approved Lists of (AL) Bacterial Names. [Volume:page (AL)] Numerical reference with asterisk Acidiphilium cryptum 31:331* original publication in the IJSB or IJSEM [Volume:page of description*] Numerical reference without Acetomicrobium faecale 38:136 Validation List in the IJSB or IJSEM asterisk [Volume:page] ≡ Acetobacter methanolicus homotypic (formerly: objective) (basonym) ≡ Acidomonas synonym; the original name is methanolica indicated as a basonym a,g = Brevibacterium albidum (as heterotypic (formerly: subjective) synonym) = synonym; the name published Curtobacterium albidum first (Curtobacterium albidum) has priority over Brevibacterium albidum a,g corrig. -

Genome Sequence of Caballeronia Sordidicola Strain PAMC 26577 Isolated from Cladonia Sp., an Arctic Lichen Species
Korean Journal of Microbiology (2017) Vol. 53, No. 2, pp. 141-143 pISSN 0440-2413 DOI https://doi.org/10.7845/kjm.2017.7037 eISSN 2383-9902 Copyright ⓒ 2017, The Microbiological Society of Korea Note (Genome Announcement) Genome sequence of Caballeronia sordidicola strain PAMC 26577 isolated from Cladonia sp., an Arctic lichen species Jhung Ahn Yang1, Soon Gyu Hong2, and Hyun-Myung Oh1* 1Department of Marine-Bio Convergence Science, Specialized Graduate School Science & Technology Convergence, Pukyong National University, Busan 48547, Republic of Korea 2Division of Polar Life Sciences, Korea Polar Research Institute, Incheon 21990, Republic of Korea 북극 지의류 Cladonia 종에서 분리한 Caballeronia sordidicola 균주 PAMC 26577의 유전체 서열 분석 양정안1 ・ 홍순규2 ・ 오현명1* 1부경대학교 과학기술융합전문대학원, 2극지연구소 (Received June 8, 2017; Accepted June 8, 2017) Caballeronia sordidicola strain PAMC 26577 was isolated Cultivable sixty eight isolates from polar lichen species from Cladonia sp., a lichen collected from Svalbard Archipelago were investigated by 16S rRNA phylogenetic analysis and they in the Arctic Ocean. Draft genomic sequences of PAMC 26577 were affiliated with the phyla Actinobacteria, Bacteroidetes, were determined using Illumina and 182 contigs were submitted Deinococcus-Thermus, and Firmicutes and with the classes to GenBank and N50 value was 159,226. The genome of PAMC Alphaproteobacteria, Betaproteobacteria, and Gammaproteo- 26577 was comprised of 8,334,211 base pairs and %G+C bacteria et al. content was 59.4. The genome included 8 ribosomal RNA genes (Lee , 2014). The strain PAMC 26577 was and 51 tRNA genes as non-coding sequences. Protein-coding originally deposited at Polar and Alpine Microbial Collection genes were 8,065 in number and they included central meta- (Lee et al., 2012) and it was reported that the strain PAMC bolism genes as well as butanol/butyrate biosynthesis, poly- 26577 was identified as Burkholderia sordidicola in the class hydroxybutyrate metabolism, serine cycle methylotrophy genes, Betaproteobacteria based on 16S rRNA sequence analysis, and glycogen metabolism. -
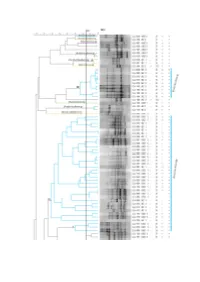
Supplementary File 1 (PDF, 1566 Kib)
Figure S1. Dendrogram based on cluster analysis of fingerprinting PCR profiles of the isolates from A. longifolia nodules, using the Pearson correlation coefficient and the unweighted pair-group method with arithmetic mean algorithm (UPGMA). 84% was the cut-off level below which isolates could be considered different. On the right are represented: isolate identification (CJJ xxx), zone from where it was isolated (UBZ/BZ x), Gram test result, morphology (rods (B) or cocci (CC)), catalase test result and oxidase test result, both (+) or (-). Colours are according to the phylum/class into each genus belong to: Proteobacteria/α-proteobacteria (blue), Proteobacteria/β-proteobacteria (orange), Proteobacteria/γ-proteobacteria (green), firmicutes/Bacilli (purple) and actinobacteria/Actinobacteria (yellow). Roman numbering identifies clusters. Isolates are identified up to genus level. Table S1. Identification of bacterial isolates obtained from unburnt and burnt zones by BLAST analysis of the 16S rRNA gene sequences. GenBank accession numbers are also indicated. GenBank % Pairwise Isolate ID Identification accession Closest hit by BLAST analysis1 Identity numbers CJJ 003 Bradyrhizobium sp. MT465339 Bradyrhizobium sp. MH612954 100% CJJ 008 Bradyrhizobium sp. MT465340 Bradyrhizobium sp. Z94815 100% CJJ 010 Bradyrhizobium sp. MT465341 Bradyrhizobium cytisi MK370569 98.1% CJJ 013 Bradyrhizobium pachyrhizi MT465342 Bradyrhizobium pachyrhizi KP769443 100% CJJ 017 Bradyrhizobium sp. MT465343 Bradyrhizobium cytisi MK370569 99.3% CJJ 020 Bradyrhizobium sp. MT465344 Bradyrhizobium sp. DQ202229 99.5% CJJ 024 Bradyrhizobium cytisi MT465345 Bradyrhizobium cytisi MK30569 100% CJJ 025 Paraburkholderia phytofirmans MT465346 Paraburkholderia phytofirmans NR_102845 100% Bradyrhizobium ganzhouense NR_133706 99.8% CJJ 026 Bradyrhizobium sp. MT465347 Bradyrhizobium rifense NR_116361 99.8% CJJ 027 Althererythrobacter sp. MT465348 Althererythrobacter sp.