Draft Genome Sequence of Caballeronia Jiangsuensis EK, a Phosphate- Solubilizing Bacterium Isolated from the Rhizosphere of Reed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multilayered Horizontal Operon Transfers from Bacteria Reconstruct a Thiamine Salvage Pathway in Yeasts
Multilayered horizontal operon transfers from bacteria reconstruct a thiamine salvage pathway in yeasts Carla Gonçalvesa and Paula Gonçalvesa,1 aApplied Molecular Biosciences Unit-UCIBIO, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal Edited by Edward F. DeLong, University of Hawaii at Manoa, Honolulu, HI, and approved September 22, 2019 (received for review June 14, 2019) Horizontal acquisition of bacterial genes is presently recognized as nisms presumed to have facilitated a transition from bacterial an important contribution to the adaptation and evolution of operon transcription to eukaryotic-style gene expression were eukaryotic genomes. However, the mechanisms underlying ex- proposed, such as gene fusion giving rise to multifunctional pro- pression and consequent selection and fixation of the prokaryotic teins (6, 23, 24), increase in intergenic distances between genes to genes in the new eukaryotic setting are largely unknown. Here we generate room for eukaryotic promoters, and independent tran- show that genes composing the pathway for the synthesis of the scription producing mRNAs with poly(A) tails have been dem- essential vitamin B1 (thiamine) were lost in an ancestor of a yeast onstrated (22). In the best documented study, which concerns a lineage, the Wickerhamiella/Starmerella (W/S) clade, known to bacterial siderophore biosynthesis operon acquired by yeasts be- harbor an unusually large number of genes of alien origin. The longing to the Wickerhamiella/Starmerella (W/S) clade, the bacte- thiamine pathway was subsequently reassembled, at least twice, rial genes acquired as an operon were shown to be functional (22). by multiple HGT events from different bacterial donors involving Thiamine, commonly known as vitamin B1, is essential for all both single genes and entire operons. -

Short-Interval Reburns in the Boreal Forest Alter Soil Bacterial Communities, Reflecting Increased Ph and Poor Conifer Seedling
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.31.437944; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Short-interval reburns in the boreal forest alter soil bacterial communities, reflecting 2 increased pH and poor conifer seedling establishment 3 4 Jamie Wooleta,b, Ellen Whitmanc,d, Marc-André Parisienc, Dan K. Thompsonc, Mike D. 5 Flannigand, and Thea Whitmana* 6 a. Department of Soil Science, University of Wisconsin-Madison, 1525 Observatory Dr., 7 Madison, WI, 53706, USA 8 b. Department of Forest and Rangeland Stewardship, Colorado State University, 1001 Amy Van 9 Dyken Way, Fort Collins, CO, 80521, USA 10 c. Northern Forestry Centre, Canadian Forest Service, Natural Resources Canada, 5320 122 11 Street, Edmonton, AB, T6H 3S5, Canada 12 d. Department of Renewable Resources, University of Alberta, 751 General Services Building, 13 Edmonton, AB, T6G 2H1, Canada 14 * Corresponding author: [email protected]; 608.263.4947 15 16 Abstract: Increasing burn rates (percentage area burned annually) in some biomes are leading to 17 fires burning in close succession, triggering rapid vegetation change as well as altering soil 18 properties. Despite the importance of soil microbes for nutrient cycling and as plant symbionts, 19 the effects of increased fire frequency on belowground microbial communities remain largely 20 unknown. We present a study of the effects of short interval reburns (defined here as <20 years 21 between fires) on soil bacterial communities in the boreal forest of northwestern Canada, using a 22 paired site design that spans wetlands and uplands, with 50 sites total. -

The Potato Yam Phyllosphere Ectosymbiont Paraburkholderia Sp
fmicb-11-00581 April 19, 2020 Time: 8:50 # 1 ORIGINAL RESEARCH published: 21 April 2020 doi: 10.3389/fmicb.2020.00581 The Potato Yam Phyllosphere Ectosymbiont Paraburkholderia sp. Msb3 Is a Potent Growth Promotor in Tomato Johannes B. Herpell1, Florian Schindler1, Mersad Bejtovic´ 1, Lena Fragner1,2, Bocar Diallo1, Anke Bellaire3, Susanne Kublik4, Bärbel U. Foesel4, Silvia Gschwendtner4, Melina Kerou5, Michael Schloter4 and Wolfram Weckwerth1,2* 1 Molecular Systems Biology (MOSYS), Department of Functional and Evolutionary Ecology, University of Vienna, Vienna, Austria, 2 Vienna Metabolomics Center (VIME), University of Vienna, Vienna, Austria, 3 Division of Structural and Functional Botany, Department of Botany and Biodiversity Research, University of Vienna, Vienna, Austria, 4 Research Unit Edited by: for Comparative Microbiome Analysis, German Research Center for Environmental Health, Helmholtz Zentrum München, 5 Michele Perazzolli, Neuherberg, Germany, Archaea Biology and Ecogenomics Division, Department of Functional and Evolutionary Ecology, University of Trento, Italy University of Vienna, Vienna, Austria Reviewed by: Stéphane Compant, The genus Paraburkholderia includes a variety of species with promising features for Austrian Institute of Technology (AIT), sustainable biotechnological solutions in agriculture through increasing crop productivity. Austria Lionel Moulin, Here, we present a novel Paraburkholderia isolate, a permanent and predominant Institut de Recherche Pour le member of the Dioscoreae bulbifera (yam family, Dioscoreaceae) -

LIST of PROKARYOTIC NAMES VALIDLY PUBLISHED in August 2016
Leibniz‐Institut DSMZ‐Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH LIST OF PROKARYOTIC NAMES VALIDLY PUBLISHED in August 2016 compiled by Dorothea Gleim, Leibniz‐Institut DSMZ ‐ Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH Braunschweig, Germany PROKARYOTIC NOMENCLATURE, Update 08/2016 2 Notes This compilation of validly published names of Prokaryotes is produced to the best of our knowledge. Nevertheless we do not accept any responsibility for errors, inaccuracies or omissions. Names of prokaryotes are defined as being validly published by the International Code of Nomenclature of Bacteria a,b. Validly published are all names which are included in the Approved Lists of Bacterial Names c,d,e,f and the names subsequently published in the International Journal of Systematic Bacteriology (IJSB) and, from January 2000, in the International Journal of Systematic and Evolutionary Microbiology (IJSEM) in the form of original articles or in the Validation Lists. Names not considered to be validly published, should no longer be used, or used in quotation marks, i.e. “Streptococcus equisimilis” to denote that the name has no standing in nomenclature. Please note that such names cannot be retrieved in this list. Explanations, Examples Numerical reference followed by Streptomyces setonii 30:401 (AL) Included in The Approved Lists of (AL) Bacterial Names. [Volume:page (AL)] Numerical reference with asterisk Acidiphilium cryptum 31:331* original publication in the IJSB or IJSEM [Volume:page of description*] Numerical reference without Acetomicrobium faecale 38:136 Validation List in the IJSB or IJSEM asterisk [Volume:page] ≡ Acetobacter methanolicus homotypic (formerly: objective) (basonym) ≡ Acidomonas synonym; the original name is methanolica indicated as a basonym a,g = Brevibacterium albidum (as heterotypic (formerly: subjective) synonym) = synonym; the name published Curtobacterium albidum first (Curtobacterium albidum) has priority over Brevibacterium albidum a,g corrig. -

Genome Sequence of Caballeronia Sordidicola Strain PAMC 26577 Isolated from Cladonia Sp., an Arctic Lichen Species
Korean Journal of Microbiology (2017) Vol. 53, No. 2, pp. 141-143 pISSN 0440-2413 DOI https://doi.org/10.7845/kjm.2017.7037 eISSN 2383-9902 Copyright ⓒ 2017, The Microbiological Society of Korea Note (Genome Announcement) Genome sequence of Caballeronia sordidicola strain PAMC 26577 isolated from Cladonia sp., an Arctic lichen species Jhung Ahn Yang1, Soon Gyu Hong2, and Hyun-Myung Oh1* 1Department of Marine-Bio Convergence Science, Specialized Graduate School Science & Technology Convergence, Pukyong National University, Busan 48547, Republic of Korea 2Division of Polar Life Sciences, Korea Polar Research Institute, Incheon 21990, Republic of Korea 북극 지의류 Cladonia 종에서 분리한 Caballeronia sordidicola 균주 PAMC 26577의 유전체 서열 분석 양정안1 ・ 홍순규2 ・ 오현명1* 1부경대학교 과학기술융합전문대학원, 2극지연구소 (Received June 8, 2017; Accepted June 8, 2017) Caballeronia sordidicola strain PAMC 26577 was isolated Cultivable sixty eight isolates from polar lichen species from Cladonia sp., a lichen collected from Svalbard Archipelago were investigated by 16S rRNA phylogenetic analysis and they in the Arctic Ocean. Draft genomic sequences of PAMC 26577 were affiliated with the phyla Actinobacteria, Bacteroidetes, were determined using Illumina and 182 contigs were submitted Deinococcus-Thermus, and Firmicutes and with the classes to GenBank and N50 value was 159,226. The genome of PAMC Alphaproteobacteria, Betaproteobacteria, and Gammaproteo- 26577 was comprised of 8,334,211 base pairs and %G+C bacteria et al. content was 59.4. The genome included 8 ribosomal RNA genes (Lee , 2014). The strain PAMC 26577 was and 51 tRNA genes as non-coding sequences. Protein-coding originally deposited at Polar and Alpine Microbial Collection genes were 8,065 in number and they included central meta- (Lee et al., 2012) and it was reported that the strain PAMC bolism genes as well as butanol/butyrate biosynthesis, poly- 26577 was identified as Burkholderia sordidicola in the class hydroxybutyrate metabolism, serine cycle methylotrophy genes, Betaproteobacteria based on 16S rRNA sequence analysis, and glycogen metabolism. -
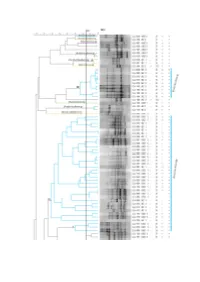
Supplementary File 1 (PDF, 1566 Kib)
Figure S1. Dendrogram based on cluster analysis of fingerprinting PCR profiles of the isolates from A. longifolia nodules, using the Pearson correlation coefficient and the unweighted pair-group method with arithmetic mean algorithm (UPGMA). 84% was the cut-off level below which isolates could be considered different. On the right are represented: isolate identification (CJJ xxx), zone from where it was isolated (UBZ/BZ x), Gram test result, morphology (rods (B) or cocci (CC)), catalase test result and oxidase test result, both (+) or (-). Colours are according to the phylum/class into each genus belong to: Proteobacteria/α-proteobacteria (blue), Proteobacteria/β-proteobacteria (orange), Proteobacteria/γ-proteobacteria (green), firmicutes/Bacilli (purple) and actinobacteria/Actinobacteria (yellow). Roman numbering identifies clusters. Isolates are identified up to genus level. Table S1. Identification of bacterial isolates obtained from unburnt and burnt zones by BLAST analysis of the 16S rRNA gene sequences. GenBank accession numbers are also indicated. GenBank % Pairwise Isolate ID Identification accession Closest hit by BLAST analysis1 Identity numbers CJJ 003 Bradyrhizobium sp. MT465339 Bradyrhizobium sp. MH612954 100% CJJ 008 Bradyrhizobium sp. MT465340 Bradyrhizobium sp. Z94815 100% CJJ 010 Bradyrhizobium sp. MT465341 Bradyrhizobium cytisi MK370569 98.1% CJJ 013 Bradyrhizobium pachyrhizi MT465342 Bradyrhizobium pachyrhizi KP769443 100% CJJ 017 Bradyrhizobium sp. MT465343 Bradyrhizobium cytisi MK370569 99.3% CJJ 020 Bradyrhizobium sp. MT465344 Bradyrhizobium sp. DQ202229 99.5% CJJ 024 Bradyrhizobium cytisi MT465345 Bradyrhizobium cytisi MK30569 100% CJJ 025 Paraburkholderia phytofirmans MT465346 Paraburkholderia phytofirmans NR_102845 100% Bradyrhizobium ganzhouense NR_133706 99.8% CJJ 026 Bradyrhizobium sp. MT465347 Bradyrhizobium rifense NR_116361 99.8% CJJ 027 Althererythrobacter sp. MT465348 Althererythrobacter sp. -

A Report of 39 Unrecorded Bacterial Species in Korea Belonging to the Classes Betaproteobacteria and Gammaproteobacteria Isolated in 2018
Journal346 of Species Research 9(4):346-361, 2020JOURNAL OF SPECIES RESEARCH Vol. 9, No. 4 A report of 39 unrecorded bacterial species in Korea belonging to the classes Betaproteobacteria and Gammaproteobacteria isolated in 2018 Yong-Seok Kim1, Hana Yi2, Myung Kyum Kim3, Chi-Nam Seong4, Wonyong Kim5, Che Ok Jeon6, Seung-Bum Kim7, Wan-Taek Im8, Kiseong Joh9 and Chang-Jun Cha1,* 1Department of Systems Biotechnology, Chung-Ang University, Anseong 17546, Republic of Korea 2Department of Public Health Sciencs & Guro Hospital, Korea University, Seoul 02841, Republic of Korea 3Department of Bio and Environmental Technology, College of Natural Science, Seoul Women’s University, Seoul 01797, Republic of Korea 4Department of Biology, Sunchon National University, Suncheon 57922, Republic of Korea 5Department of Microbiology, Chung-Ang University College of Medicine, Seoul 06974, Republic of Korea 6Department of Life Science, Chung-Ang University, Seoul 06974, Republic of Korea 7Department of Microbiology and Molecular Biology, Chungnam National University, Daejeon 34134, Republic of Korea 8Department of Biotechnology, Hankyoung National University, Anseong 17579, Rpublic of Korea 9Department of Bioscience and Biotechnology, Hankuk University of Foreign Studies, Gyeonggi 17035, Republic of Korea *Correspondent: [email protected] In the project of a comprehensive investigation of indigenous prokaryotic species in Korea, a total of 39 bacterial strains phylogenetically belonging to the classes Betaproteobacteria and Gammaproteobacteria were isolated from various environmental sources such as soil, cultivated soil, sludge, seawater, marine sediment, algae, human, tree, moss, tidal flat, beach sand and lagoon. Phylogenetic analysis based on 16S rRNA gene sequences revealed that 39 strains showed the high sequence similarities (≥98.7%) to the closest type strains and formed robust phylogenetic clades with closely related species in the classes Betaproteobacteria and Gammaproteobacteria. -

Horizontal Gene Transfer to a Defensive Symbiont with a Reduced Genome
bioRxiv preprint doi: https://doi.org/10.1101/780619; this version posted September 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Horizontal gene transfer to a defensive symbiont with a reduced genome 2 amongst a multipartite beetle microbiome 3 Samantha C. Waterwortha, Laura V. Flórezb, Evan R. Reesa, Christian Hertweckc,d, 4 Martin Kaltenpothb and Jason C. Kwana# 5 6 Division of Pharmaceutical Sciences, School of Pharmacy, University of Wisconsin- 7 Madison, Madison, Wisconsin, USAa 8 Department of Evolutionary Ecology, Institute of Organismic and Molecular Evolution, 9 Johannes Gutenburg University, Mainz, Germanyb 10 Department of Biomolecular Chemistry, Leibniz Institute for Natural Products Research 11 and Infection Biology, Jena, Germanyc 12 Department of Natural Product Chemistry, Friedrich Schiller University, Jena, Germanyd 13 14 #Address correspondence to Jason C. Kwan, [email protected] 15 16 17 18 1 bioRxiv preprint doi: https://doi.org/10.1101/780619; this version posted September 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 19 ABSTRACT 20 The loss of functions required for independent life when living within a host gives rise to 21 reduced genomes in obligate bacterial symbionts. Although this phenomenon can be 22 explained by existing evolutionary models, its initiation is not well understood. -

Structural and Functional Differentiation of Bacterial Communities
www.nature.com/scientificreports OPEN Structural and functional diferentiation of bacterial communities in post-coal mining reclamation soils of South Africa: bioindicators of soil ecosystem restoration Obinna T. Ezeokoli1,2,3, Cornelius C. Bezuidenhout1, Mark S. Maboeta1, Damase P. Khasa3,4 & Rasheed A. Adeleke1,2* Soil microbial communities are suitable soil ecosystem health indicators due to their sensitivity to management practices and role in soil ecosystem processes. Presently, information on structural and functional diferentiation of bacterial communities in post-coal mining reclamation soils of South Africa is sparse. Here, bacterial communities in three post-coal mining reclamation soils were investigated using community-level physiological profling (CLPP), enzyme activities, and next-generation sequencing of 16S rRNA gene. Inferences were drawn in reference to adjacent unmined soils. CLPP- based species diversity and proportionality did not difer signifcantly (P > 0.05) whereas activities of β-glucosidase, urease and phosphatases were signifcantly (P < 0.05) infuenced by site and soil history (reclaimed vs unmined). Bacterial communities were infuenced (PERMANOVA, P < 0.05) by soil history and site diferences, with several phylotypes diferentially abundant between soils. Contrastingly, predicted functional capabilities of bacterial communities were not diferent (PERMANOVA, P > 0.05), suggesting redundancy in bacterial community functions between reclamation and unmined soils. Silt content, bulk density, pH, electrical conductivity, Na and Ca signifcantly infuenced soil bacterial communities. Overall, results indicate that bacterial community structure refects underlying diferences between soil ecosystems, and suggest the restoration of bacterial diversity and functions over chronological age in reclamation soils. Te soil ecosystem supports numerous interactions between living and non-living matter. -

Phenolic Acid-Degrading Paraburkholderia Prime Decomposition in Forest Soil
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.317347; this version posted September 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Phenolic acid-degrading Paraburkholderia prime decomposition in forest soil Roland C. Wilhelm1†*, Christopher M. DeRito2†, James P. Shapleigh2, Eugene L. Madsen2∞ and Daniel H. Buckley1 1 School of Integrative Plant Science, Bradfield Hall, Cornell University, Ithaca, NY, 14853, USA 2 Department of Microbiology, Wing Hall, Cornell University, Ithaca, NY, 14853, USA † These authors contributed equally to the work. ∞ Deceased August 9th, 2017 *Corresponding Author: Dr. Roland C. Wilhelm Current address: School of Integrative Plant Science, 306 Tower Road, Cornell University, Ithaca, NY 14853 Telephone: +1 607-255-1716 E-mail: [email protected] Running Title: “Phenolic acid-degrading bacteria and soil priming” Keywords: soil ecology, carbon cycling, priming effect, Paraburkholderia, forest soil, stable isotope probing, p-hydroxybenzoic acid, pobA. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.317347; this version posted September 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract 1 Plant-derived phenolic acids are metabolized by soil microorganisms whose activity may 2 enhance the decomposition of soil organic carbon (SOC). We characterized whether phenolic 3 acid-degrading bacteria would enhance SOC mineralization in forest soils when primed with 13C- 4 labeled p-hydroxybenzoic acid (PHB). We further investigated whether PHB-induced priming 5 could explain differences in SOC content among mono-specific tree plantations in a 70-year-old 6 common garden experiment. -

Supplemental Table 1
Supplemental Table 1: Sample No. Raw Reads No. Reads in Final Read Length in Final Analysis Analysis Min. Avg. Max. SB33-m1 127,976 101,129 SB34-m1 135,311 99,738 SB35-m1 129,943 105,604 SB36-m1 148,449 110,127 SB32-m4 131,497 106,878 SB33-m4 157,265 121,165 SB34-m4 95,709 75,902 SB35-m4 78,500 61,120 SB36-m4 123,032 92,575 SB74-wg 89,147 70,057 SB80-wg 203,580 156,361 SB84-wg 138,314 105,484 SB85-wg 127,780 101,425 SB86-wg 142,729 109,106 Total: 1,829,232 1,416,671 Supplemental Table 1. Caballeronia spp. bacteria isolated from Anasa tristis. Location abbreviations indicate: BCI - BCI Research Laboratory, Columbia, Missouri; CRY - Crystal Organic Farms, Newborn, Georgia; DEC - DeCamp Gardens, Albion, Indiana; FFA - Farlow Farm, Archdale, Indiana; FFF - Front Field Farm, Winterville, Georgia; MGA - Mission Garden, Tucson, Arizona; MLF - Merry Lea Farm, Albion, Indiana; NDG - North Dekalb Garden, Atlanta, Georgia; OAK - Oakhurst Community Garden, Decatur, Georgia; OXF - Oxford Farm, Oxford, Georgia; SBG - Stoy/Barse Gardens, Auburn, Indiana; TMF - Ten Mothers Farm, Hillsborough, North Carolina; UFL – University of Florida Garden, Gainesville, Georgia; and WOG - Woodland Gardens, Atlanta, Georgia. All were collected within the United States. Classification and % match to genus are based on the Ribosomal Database Project (RDP) classifier. Isolate Name Individual Life Stage Tissue State Site NCBI Family Genus % Match to Genus 3_56_M4_b 3.56 3 M4 crypt Georgia CRY KX239758 Burkholderiaceae Caballeronia 100 3_56_M4_c 3.56 3 M4 crypt Georgia -

Draft Genome of Paraburkholderia Caballeronis Tne-841T, a Free-Living
Rojas-Rojas et al. Standards in Genomic Sciences (2017) 12:80 DOI 10.1186/s40793-017-0294-7 SHORT GENOME REPORT Open Access Draft genome of Paraburkholderia caballeronis TNe-841T, a free-living, nitrogen-fixing, tomato plant-associated bacterium Fernando Uriel Rojas-Rojas1, Erika Yanet Tapia-García1, Maskit Maymon2, Ethan Humm2, Marcel Huntemann3, Alicia Clum3, Manoj Pillay3, Krishnaveni Palaniappan3, Neha Varghese3, Natalia Mikhailova3, Dimitrios Stamatis3, T. B. K. Reddy3, Victor Markowitz3, Natalia Ivanova3, Nikos Kyrpides3, Tanja Woyke3, Nicole Shapiro3, Ann M. Hirsch2,4 and Paulina Estrada-de los Santos1* Abstract Paraburkholderia caballeronis is a plant-associated bacterium. Strain TNe-841T was isolated from the rhizosphere of tomato (Solanum lycopersicum L. var. lycopersicum) growing in Nepantla Mexico State. Initially this bacterium was found to effectively nodulate Phaseolus vulgaris L. However, from an analysis of the genome of strain TNe-841T and from repeat inoculation experiments, we found that this strain did not nodulate bean and also lacked nodulation genes, suggesting that the genes were lost. The genome consists of 7,115,141 bp with a G + C content of 67.01%. The sequence includes 6251 protein-coding genes and 87 RNA genes. Keywords: Paraburkholderia caballeronis, Tomato plant, Rhizosphere, Nitrogen fixation, Root nodulation Introduction ThegenomesequenceofP. caballeronis TNe-841T was Paraburkholderia caballeronis was isolated in the State obtained in cooperation with JGI-DOE. The type species is of Mexico, Mexico from the tomato rhizosphere as a TNe-841T (= LMG 26416T = CIP 110324T). free-living, nitrogen-fixing bacterial species [1]. It was described as B. caballeronis and found to nodulate Phaseolus vulgaris L. [2].