Jellyfish Care Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project
High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project AEA Technology, Environment Contract: W/35/00632/00/00 For: The Department of Trade and Industry New & Renewable Energy Programme Report issued 30 August 2002 (Version with minor corrections 16 September 2002) Keith Hiscock, Harvey Tyler-Walters and Hugh Jones Reference: Hiscock, K., Tyler-Walters, H. & Jones, H. 2002. High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Report from the Marine Biological Association to The Department of Trade and Industry New & Renewable Energy Programme. (AEA Technology, Environment Contract: W/35/00632/00/00.) Correspondence: Dr. K. Hiscock, The Laboratory, Citadel Hill, Plymouth, PL1 2PB. [email protected] High level environmental screening study for offshore wind farm developments – marine habitats and species ii High level environmental screening study for offshore wind farm developments – marine habitats and species Title: High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Contract Report: W/35/00632/00/00. Client: Department of Trade and Industry (New & Renewable Energy Programme) Contract management: AEA Technology, Environment. Date of contract issue: 22/07/2002 Level of report issue: Final Confidentiality: Distribution at discretion of DTI before Consultation report published then no restriction. Distribution: Two copies and electronic file to DTI (Mr S. Payne, Offshore Renewables Planning). One copy to MBA library. Prepared by: Dr. K. Hiscock, Dr. H. Tyler-Walters & Hugh Jones Authorization: Project Director: Dr. Keith Hiscock Date: Signature: MBA Director: Prof. S. Hawkins Date: Signature: This report can be referred to as follows: Hiscock, K., Tyler-Walters, H. -

Research Funding (Total $2,552,481) $15,000 2019
CURRICULUM VITAE TENNESSEE AQUARIUM CONSERVATION INSTITUTE 175 BAYLOR SCHOOL RD CHATTANOOGA, TN 37405 RESEARCH FUNDING (TOTAL $2,552,481) $15,000 2019. Global Wildlife Conservation. Rediscovering the critically endangered Syr-Darya Shovelnose Sturgeon. $10,000 2019. Tennessee Wildlife Resources Agency. Propagation of the Common Logperch as a host for endangered mussel larvae. $8,420 2019. Tennessee Wildlife Resources Agency. Monitoring for the Laurel Dace. $4,417 2019. Tennessee Wildlife Resources Agency. Examining interactions between Laurel Dace (Chrosomus saylori) and sunfish $12,670 2019. Trout Unlimited. Southern Appalachian Brook Trout propagation for reintroduction to Shell Creek. $106,851 2019. Private Donation. Microplastic accumulation in fishes of the southeast. $1,471. 2019. AZFA-Clark Waldram Conservation Grant. Mayfly propagation for captive propagation programs. $20,000. 2019. Tennessee Valley Authority. Assessment of genetic diversity within Blotchside Logperch. $25,000. 2019. Riverview Foundation. Launching Hidden Rivers in the Southeast. $11,170. 2018. Trout Unlimited. Propagation of Southern Appalachian Brook Trout for Supplemental Reintroduction. $1,471. 2018. AZFA Clark Waldram Conservation Grant. Climate Change Impacts on Headwater Stream Vertebrates in Southeastern United States $1,000. 2018. Hamilton County Health Department. Step 1 Teaching Garden Grants for Sequoyah School Garden. $41,000. 2018. Riverview Foundation. River Teachers: Workshops for Educators. $1,000. 2018. Tennessee Valley Authority. Youth Freshwater Summit $20,000. 2017. Tennessee Valley Authority. Lake Sturgeon Propagation. $7,500 2017. Trout Unlimited. Brook Trout Propagation. $24,783. 2017. Tennessee Wildlife Resource Agency. Assessment of Percina macrocephala and Etheostoma cinereum populations within the Duck River Basin. $35,000. 2017. U.S. Fish and Wildlife Service. Status surveys for conservation status of Ashy (Etheostoma cinereum) and Redlips (Etheostoma maydeni) Darters. -

Treatment of Lion´S Mane Jellyfish Stings- Hot Water Immersion Versus Topical Corticosteroids
THE SAHLGRENSKA ACADEMY Treatment of Lion´s Mane jellyfish stings- hot water immersion versus topical corticosteroids Degree Project in Medicine Anna Nordesjö Programme in Medicine Gothenburg, Sweden 2016 Supervisor: Kai Knudsen Department of Anesthesia and Intensive Care Medicine 1 CONTENTS Abstract ................................................................................................................................................... 3 Introduction ............................................................................................................................................. 3 Background ............................................................................................................................................. 4 Jellyfish ............................................................................................................................................... 4 Anatomy .......................................................................................................................................... 4 Nematocysts .................................................................................................................................... 4 Jellyfish in Scandinavian waters ......................................................................................................... 5 Lion’s Mane jellyfish, Cyanea capillata .......................................................................................... 5 Moon jelly, Aurelia aurita .............................................................................................................. -

Anthopleura and the Phylogeny of Actinioidea (Cnidaria: Anthozoa: Actiniaria)
Org Divers Evol (2017) 17:545–564 DOI 10.1007/s13127-017-0326-6 ORIGINAL ARTICLE Anthopleura and the phylogeny of Actinioidea (Cnidaria: Anthozoa: Actiniaria) M. Daly1 & L. M. Crowley2 & P. Larson1 & E. Rodríguez2 & E. Heestand Saucier1,3 & D. G. Fautin4 Received: 29 November 2016 /Accepted: 2 March 2017 /Published online: 27 April 2017 # Gesellschaft für Biologische Systematik 2017 Abstract Members of the sea anemone genus Anthopleura by the discovery that acrorhagi and verrucae are are familiar constituents of rocky intertidal communities. pleisiomorphic for the subset of Actinioidea studied. Despite its familiarity and the number of studies that use its members to understand ecological or biological phe- Keywords Anthopleura . Actinioidea . Cnidaria . Verrucae . nomena, the diversity and phylogeny of this group are poor- Acrorhagi . Pseudoacrorhagi . Atomized coding ly understood. Many of the taxonomic and phylogenetic problems stem from problems with the documentation and interpretation of acrorhagi and verrucae, the two features Anthopleura Duchassaing de Fonbressin and Michelotti, 1860 that are used to recognize members of Anthopleura.These (Cnidaria: Anthozoa: Actiniaria: Actiniidae) is one of the most anatomical features have a broad distribution within the familiar and well-known genera of sea anemones. Its members superfamily Actinioidea, and their occurrence and exclu- are found in both temperate and tropical rocky intertidal hab- sivity are not clear. We use DNA sequences from the nu- itats and are abundant and species-rich when present (e.g., cleus and mitochondrion and cladistic analysis of verrucae Stephenson 1935; Stephenson and Stephenson 1972; and acrorhagi to test the monophyly of Anthopleura and to England 1992; Pearse and Francis 2000). -

Pdf) and Their Values Are Plotted Against Temperature in Fig
Vol. 510: 255–263, 2014 MARINE ECOLOGY PROGRESS SERIES Published September 9 doi: 10.3354/meps10799 Mar Ecol Prog Ser Contribution to the Theme Section ‘Jellyfish blooms and ecological interactions’ FREEREE ACCESSCCESS Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita Zhilu Fu1, Masashi Shibata1, Ryosuke Makabe2, Hideki Ikeda1, Shin-ichi Uye1,* 1Graduate School of Biosphere Science, Hiroshima University, 4-4 Kagamiyama 1 Chome, Higashi-Hiroshima 739−8528, Japan 2Faculty of Science and Engineering, Ishinomaki Senshu University, 1 Shinmito Minamisakai, Ishinomaki 986-8580, Japan ABSTRACT: Scyphozoan ephyrae need to start feeding before their endogenous nutritional reserves run out, and the success of feeding and growth is crucial to their recruitment into the medusa population. To evaluate starvation resistance in first-feeding ephyrae of the moon jellyfish Aurelia aurita s.l., we determined their point of no return (PNR50), i.e. days of starvation after which 50% of ephyrae die even if they then feed. PNR50 values were 33.8, 38.4 and 58.6 d at 15, 12 and 9°C, respectively. Before reaching PNR50, the ephyrae showed significant body size reduc- tion: ca. 30 and 50% decrease in disc diameter and carbon content, respectively. These PNR50 val- ues are nearly 1 order of magnitude longer than those of larval marine molluscs, crustaceans and fishes, which is attributable to the ephyra’s extremely low metabolic (i.e. respiration) rate relative to its copious carbon reserves. Such a strong endurance under prolonged starvation is likely an adaptive strategy for A. aurita ephyrae, the release of which is programmed to occur during the annual period of lowest temperatures, allowing them to cope with the concomitant seasonal food scarcity. -

The Jellyfish Fishery in Mexico
Vol.4, No.6A, 57-61 (2013) Agricultural Sciences http://dx.doi.org/10.4236/as.2013.46A009 The jellyfish fishery in Mexico Juana López-Martínez*, Javier Álvarez-Tello Centro de Investigaciones Biológicas del Noroeste, S.C. (CIBNOR), Unidad Sonora, Campus Guaymas, Guaymas, México; *Corresponding Author: [email protected] Received 26 April 2013; revised 26 May 2013; accepted 15 June 2013 Copyright © 2013 Juana López-Martínez, Javier Álvarez-Tello. This is an open access article distributed under the Creative Com- mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT ure 1), because some species secrete painful neurotoxic, even deadly, venom. Globally there has been a slight Jellyfish has been captured in Asia for 1700 increase of individuals in this group of organisms during years, and it has been considered a delicacy. the last decades [1], but in some countries as Australia, Since the 70s important jellyfish fisheries have jellyfish is considered a plague, so in response the gov- developed in several parts of the world, with ernment developed programs to control them. Among the catches increasing exponentially, reaching possible causes of the increase of jellyfish population, an 500,000 tons per year in the mid-nineties. In increase in water temperature due to global warming Mexico, only the cannonball jellyfish Stomolo- [2,3], reduction of predators by overfishing, and water phus meleagris is captured commercially. Most pollution [4,5] have been mentioned. Waste discharge of the capture of this jellyfish species is ob- into the sea, and in general, the increment of pollution in tained within the Gulf of California, specifically in the state of Sonora. -
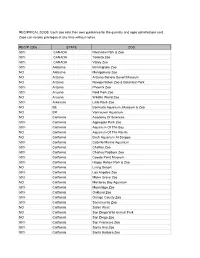
2006 Reciprocal List
RECIPRICAL ZOOS. Each zoo sets their own guidelines for the quantity and ages admitted per card. Zoos can revoke privileges at any time without notice. RECIP 2006 STATE ZOO 50% CANADA Riverview Park & Zoo 50% CANADA Toronto Zoo 50% CANADA Valley Zoo 50% Alabama Birmingham Zoo NO Alabama Montgomery Zoo NO Arizona Arizona-Sonora Desert Museum NO Arizona Navajo Nation Zoo & Botanical Park 50% Arizona Phoenix Zoo 50% Arizona Reid Park Zoo NO Arizona Wildlife World Zoo 50% Arkansas Little Rock Zoo NO BE Bermuda Aquarium, Museum & Zoo NO BR Vancouver Aquarium NO California Academy Of Sciences 50% California Applegate Park Zoo 50% California Aquarium Of The Bay NO California Aquarium Of The Pacific NO California Birch Aquarium At Scripps 50% California Cabrillo Marine Aquarium 50% California Chaffee Zoo 50% California Charles Paddock Zoo 50% California Coyote Point Museum 50% California Happy Hollow Park & Zoo NO California Living Desert 50% California Los Angeles Zoo 50% California Micke Grove Zoo NO California Monterey Bay Aquarium 50% California Moonridge Zoo 50% California Oakland Zoo 50% California Orange County Zoo 50% California Sacramento Zoo NO California Safari West NO California San Diego Wild Animal Park NO California San Diego Zoo 50% California San Francisco Zoo 50% California Santa Ana Zoo 50% California Santa Barbara Zoo NO California Seaworld San Diego 50% California Sequoia Park Zoo NO California Six Flags Marine World NO California Steinhart Aquarium NO CANADA Calgary Zoo 50% Colorado Butterfly Pavilion NO Colorado Cheyenne -

Caretta Caretta) As Revealed by Stable Isotopes and Satellite Telemetry
Mar Biol (2012) 159:1255–1267 DOI 10.1007/s00227-012-1906-9 ORIGINAL PAPER Distribution of foraging habitats of male loggerhead turtles (Caretta caretta) as revealed by stable isotopes and satellite telemetry Mariela Pajuelo · Karen A. Bjorndal · Kimberly J. Reich · Michael D. Arendt · Alan B. Bolten Received: 13 October 2011 / Accepted: 20 February 2012 / Published online: 7 March 2012 © Springer-Verlag 2012 Abstract Most studies on the foraging ecology of logger- Introduction head turtles (Caretta caretta) have focused on adult females and juveniles. Little is known about the foraging Knowledge of foraging ground distribution of highly patterns of adult male loggerheads. We analyzed tissues for migratory animals is critical for understanding their forag- carbon and nitrogen stable isotopes (13C and 15N) from ing behavior and habitat use. IdentiWcation of key habitats 29 adult male loggerheads tracked with satellite transmit- helps not only to characterize life history features of popu- ters from one breeding area in Florida, USA, to evaluate lations (Block et al. 2001), but also to assess the impact of their foraging habitats in the Northwest Atlantic (NWA). threats that populations may face (Hays et al. 2003). Most Our study revealed large variations in 13C and 15N and a eVorts to identify key habitats and movement patterns have correlation between both 13C and 15N and the latitude to used Xipper tags (Limpus et al. 1992), genetic markers which the loggerheads traveled after the mating season, (Bolker et al. 2007), chemical analysis (Thorrold et al. thus reXecting a geographic pattern in the isotopic signa- 2001), and electronic tagging (Block et al. -

South Carolina Department of Natural Resources
FOREWORD Abundant fish and wildlife, unbroken coastal vistas, miles of scenic rivers, swamps and mountains open to exploration, and well-tended forests and fields…these resources enhance the quality of life that makes South Carolina a place people want to call home. We know our state’s natural resources are a primary reason that individuals and businesses choose to locate here. They are drawn to the high quality natural resources that South Carolinians love and appreciate. The quality of our state’s natural resources is no accident. It is the result of hard work and sound stewardship on the part of many citizens and agencies. The 20th century brought many changes to South Carolina; some of these changes had devastating results to the land. However, people rose to the challenge of restoring our resources. Over the past several decades, deer, wood duck and wild turkey populations have been restored, striped bass populations have recovered, the bald eagle has returned and more than half a million acres of wildlife habitat has been conserved. We in South Carolina are particularly proud of our accomplishments as we prepare to celebrate, in 2006, the 100th anniversary of game and fish law enforcement and management by the state of South Carolina. Since its inception, the South Carolina Department of Natural Resources (SCDNR) has undergone several reorganizations and name changes; however, more has changed in this state than the department’s name. According to the US Census Bureau, the South Carolina’s population has almost doubled since 1950 and the majority of our citizens now live in urban areas. -

Towards the Acoustic Estimation of Jellyfish Abundance
MARINE ECOLOGY PROGRESS SERIES Vol. 295: 105–111, 2005 Published June 23 Mar Ecol Prog Ser Towards the acoustic estimation of jellyfish abundance Andrew S. Brierley1,*, David C. Boyer2, 6, Bjørn E. Axelsen3, Christopher P. Lynam1, Conrad A. J. Sparks4, Helen J. Boyer2, Mark J. Gibbons5 1Gatty Marine Laboratory, University of St. Andrews, Fife KY16 8LB, UK 2National Marine Information and Research Centre, PO Box 912, Swakopmund, Namibia 3Institute of Marine Research, PO Box 1870 Nordnes, 5817 Bergen, Norway 4Faculty of Applied Sciences, Cape Technikon, PO Box 652, Cape Town 8000, South Africa 5Zoology Department, University of Western Cape, Private Bag X 17, Bellville 7535, South Africa 6Present address: Fisheries & Environmental Research Support, Orchard Farm, Cockhill, Castle Cary, Somerset BA7 7NY, UK ABSTRACT: Acoustic target strengths (TSs) of the 2 most common large medusae, Chrysaora hysoscella and Aequorea aequorea, in the northern Benguela (off Namibia) have previously been estimated (at 18, 38 and 120 kHz) from acoustic data collected in conjunction with trawl samples, using the ‘comparison method’. These TS values may have been biased because the method took no account of acoustic backscatter from mesozooplankton. Here we report our efforts to improve upon these estimates, and to determine TS additionally at 200 kHz, by conducting additional sampling for mesozooplankton and fish larvae, and accounting for their likely contribution to the total backscatter. Published sound scattering models were used to predict the acoustic backscatter due to the observed numerical densities of mesozooplankton and fish larvae (solving the forward problem). Mean volume backscattering due to jellyfish alone was then inferred by subtracting the model-predicted values from the observed water-column total associated with jellyfish net samples. -

Pelagia Benovici Sp. Nov. (Cnidaria, Scyphozoa): a New Jellyfish in the Mediterranean Sea
Zootaxa 3794 (3): 455–468 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3794.3.7 http://zoobank.org/urn:lsid:zoobank.org:pub:3DBA821B-D43C-43E3-9E5D-8060AC2150C7 Pelagia benovici sp. nov. (Cnidaria, Scyphozoa): a new jellyfish in the Mediterranean Sea STEFANO PIRAINO1,2,5, GIORGIO AGLIERI1,2,5, LUIS MARTELL1, CARLOTTA MAZZOLDI3, VALENTINA MELLI3, GIACOMO MILISENDA1,2, SIMONETTA SCORRANO1,2 & FERDINANDO BOERO1, 2, 4 1Dipartimento di Scienze e Tecnologie Biologiche ed Ambientali, Università del Salento, 73100 Lecce, Italy 2CoNISMa, Consorzio Nazionale Interuniversitario per le Scienze del Mare, Roma 3Dipartimento di Biologia e Stazione Idrobiologica Umberto D’Ancona, Chioggia, Università di Padova. 4 CNR – Istituto di Scienze Marine, Genova 5Corresponding authors: [email protected], [email protected] Abstract A bloom of an unknown semaestome jellyfish species was recorded in the North Adriatic Sea from September 2013 to early 2014. Morphological analysis of several specimens showed distinct differences from other known semaestome spe- cies in the Mediterranean Sea and unquestionably identified them as belonging to a new pelagiid species within genus Pelagia. The new species is morphologically distinct from P. noctiluca, currently the only recognized valid species in the genus, and from other doubtful Pelagia species recorded from other areas of the world. Molecular analyses of mitochon- drial cytochrome c oxidase subunit I (COI) and nuclear 28S ribosomal DNA genes corroborate its specific distinction from P. noctiluca and other pelagiid taxa, supporting the monophyly of Pelagiidae. Thus, we describe Pelagia benovici sp. -

Cirripedia of Madeira
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade do Algarve Helgol Mar Res (2006) 60: 207–212 DOI 10.1007/s10152-006-0036-5 ORIGINAL ARTICLE Peter Wirtz Æ Ricardo Arau´jo Æ Alan J. Southward Cirripedia of Madeira Received: 13 September 2005 / Revised: 12 January 2006 / Accepted: 13 January 2006 / Published online: 3 February 2006 Ó Springer-Verlag and AWI 2006 Abstract We give a list of Cirripedia from Madeira phers. The marine invertebrates have been less studied Island and nearby deep water, based on specimens in and there has been no compilation of cirripede records the collection of the Museu Municipal do Funchal for Madeira, comparable to those for the Azores (Histo´ria Natural) (MMF), records mentioned in the archipelago (Young 1998a; Southward 1999). We here literature, and recent collections. Tesseropora atlantica summarize records from Madeira and nearby deep water Newman and Ross, 1976 is recorded from Madeira for and discuss their biogeographical implications. the first time. The Megabalanus of Madeira is M. az- oricus. There are 20 genera containing 27 species, of which 22 occur in depths less than 200 m. Of these Methods shallow water species, eight are wide-ranging oceanic forms that attach to other organisms or to floating The records are based on (1) the work of R.T. Lowe, objects, leaving just 13 truly benthic shallow water who sent specimens to Charles Darwin; (2) material in barnacles. This low diversity is probably a consequence the Museu Municipal do Funchal (Histo´ria Natural) of the distance from the continental coasts and the (MMF); (3) casual collecting carried out by residents or small area of the available habitat.