Analytical Development and Traceability in Food Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Swiss Dairy Economy in Figures 2019/20
Swiss Dairy Economy in figures 2019/20 Editor: Swiss Milk Producers SMP Weststrasse 10, P.O.Box CH-3000 Berne 6 Switzerland Telephone +41 (0)31 359 51 11 [email protected] swissmilk.ch Overview All-year businesses Change 17/18 (summering businesses excluded) 2017 2018 absolute in percent Producers number 20'372 19'735 -637 -3.1% Average delivery kg 163'019 168'967 5'948 3.6% Average size of businesses ha 26.4 26.8 0.4 1.5% Average of milk quantity sold per ha (1 ha = 2.47 acres) kg 6'171 6'303 131 2.1% Average milk supply per cow kg 6'244 6'341 96 1.5% Source: Milk statistics Dairy farms according to quantity of milk production 3'500 3'000 2'500 2'000 1'500 1'000 Number of dairy farms dairy of Number 500 0 0–50 >700 50–100 100–150 150–200 200–250 250–300 300–400 400–500 500–700 Valley areas Mountain areas Tons of milk marketed 2018 Source: TSM Treuhand GmbH Dairy farms according to size categories (2018) 2'000 1'800 1'600 1'400 1'200 1'000 800 600 Number of dairy farms dairy of Number 400 200 0 <5 5–10 10–15 15–20 20–25 25–30 30–35 35–40 40–50 50–75 >75 Valley areas Mountain areas Hectares (1 ha = 2.47 acres) productive land Source: TSM Treuhand GmbH Milk performance from cattle of the herd book Breeds 2015/16 2016/17 2017/18 Change 17/18 Standard lactation kg kg kg absolute in percent Brown cattle 7'078 7'171 7'184 13 0.2% Red Holstein 8'062 8'140 8'127 -13 -0.2% Holstein* 8'838 8'813 8'984 171 1.9% Fleckvieh cattle 7'028 7'098 7'100 2 0.0% Simmental 5'767 5'940 5'879 -61 -1.0% Montbéliarde 7'553 7'608 7'472 -136 -1.8% Jersey 5'624 5'718 -

Puur Zwitserse Kaasspecialiteiten • 100 % Zwitserland
• 100 % Zwitserland Puur Zwitserse Kaasspecialiteiten • 100 % Natuur • 100 % Genieten Raclette du Valais AOP Gewicht ca. 5 kg Rijpingstijd min. 3 maanden De rauwmelkse Raclette du Valais AOP is een 100% natuurlijk product uit de Zwitserse bergen van Wallis. Deze half harde Raclettekaas heeft een uitzonderlijke smaak en kwaliteit. Oorspronkelijk werd Raclette de Valais werd gesmolten door/voor de open haard. Deze bereidingswijze is sinds de jaren 50 vervangen door de racletteoven. Wal- liser Raclette AOP kan ook worden bereid in gourmetpannetjes. Het is echter belangrijk dat de kaas niet te veel en te lang wordt verwarmd. Der Scharfe Maxx Gewicht ca.6-7 kg Rijpingstijd ca. 5-7 maanden Der Scharfe Maxx is een aromatische en pittige kaas voor liefhebbers. Deze harde kaas is gemaakt met niet-gepas- teuriseerde volle melk. De zachte smeltende textuur en zijn unieke smaak zal uw smaakpapillen verwennen. Na de productie en een behandeling in het pekelbad, rijpt de Scharfe Maxx met regelmatige zorg uit in speciaal gecondi- tioneerde rijping kelders. Die Alter Schweizer Gewicht ca.6-7 kg Rijpingstijd ca. 10 maanden Die Alter Schweizer is een kaas uit Oost Zwitserland met een lange rijpingstijd waardoor hij een pittige fijne smaak heeft. Met deze leeftijd wordt hij zelfs wat kruimelig en is niet te scherp. Mooie unieke kaas van gethermiseerde melk uit de streek van de Bodensee. Mont Vully Gewicht ca.5-7 kg Rijpingstijd 10-20 weken Mont Vully is een halfharde kaas gemaakt met gethermiseerde melk. Deze kaas is tijdens het rijpen ingewreven met Pinot Noir uit de streek, waardoor hij een uitzonderlijke smaak krijgt en geschikt is voor iedere gelegenheid! Deze kaas is zeer geschikt als dessertkaas, een mooie zachte smaak met een bijzondere uitstraling. -

Mechanism and Control of the Eye Formation in Cheese
International Dairy Journal 47 (2015) 118e127 Contents lists available at ScienceDirect International Dairy Journal journal homepage: www.elsevier.com/locate/idairyj Mechanism and control of the eye formation in cheese * Dominik Guggisberg a, , Philipp Schuetz b, c, Hans Winkler a, Rudolf Amrein a, Ernst Jakob a, Marie-Therese Frohlich-Wyder€ a, Stefan Irmler a, Walter Bisig a, Iwan Jerjen b, Mathieu Plamondon b, Jürgen Hofmann b, Alexander Flisch b, Daniel Wechsler a a Agroscope, Institute for Food Sciences IFS, CH-3003 Bern, Switzerland b EMPA, Center for X-ray Analytics, CH-8600 Dübendorf, Switzerland c Lucerne University of Applied Sciences and Arts, CH-6048 Horw, Switzerland article info abstract Article history: The production of Swiss-type cheeses with a typical number, size, and distribution of eyes is a difficult Received 28 August 2014 task, especially when bactofuged or microfiltrated milk is utilised. In this study, the potential of mi- Received in revised form croparticles (plant origin) to influence eye formation in cheese, was assessed. Eight experimental 5 March 2015 Emmental cheeses were produced with one replicate from microfiltrated milk with addition of 0.0625 Accepted 6 March 2015 e4.000 mg of powdered hay to the milk (90 L) and ripened for 130 days. Eye formation was quantified by Available online 20 March 2015 means of X-ray computed tomography (between 30 and 130 days). The contents of fat, water, citric acid, lactic acid, and volatile carboxylic acids were determined at 130 days. The results demonstrate that microparticles of plant origin act as eye nuclei that control the number (P < 0.001) and size of the eyes in cheese in a dose-dependent manner. -

Swiss Cheese
ALP science 2008, No. 518 SWISS CHEESE Technical-scientific information Contents 1 Introduction 3 2 What factors affect eye development in Swiss cheese? 4 3 What causes «blind» Emmental cheese? 5 4 What causes irregular eye formation, slits or cracks in Emmental cheese? 6 5 What is aspartase of Propionibacterium? 7 6 How does aspartase of Propionibacterium affect Swiss cheese? 8 7 How may the size and quantity of the eyes in Emmentaltype cheese be controlled? 9 8 How do I control the elastic texture of Swiss-typ cheese? 12 9 Why does Swiss cheese have a sweet fl avour? 14 10 What are the causes of the most common fl avour defects of Swiss cheese? 14 ALP science 11 Is my Emmental cheese hygienically safe? 16 Title Swiss cheese Picture on cover Emmentaler Switzerland Published in Woodhead Publishing in Food Science, Technology and Nutrition, Cheese problems solved, 2007, [117–127], p. 246–265 Authors Marie-Therese Fröhlich-Wyder and Hans-Peter Bachmann Publisher Agroscope Liebefeld-Posieux Research Station ALP Schwarzenburgstrasse 161 CH-3003 Berne Telefon +41 (0)31 323 84 18 Fax +41 (0)31 323 82 27 http: www.alp.admin.ch e-mail: [email protected] Contact Marie-Therese Fröhlich-Wyder, ALP Telefon +41 (0)31 323 82 23 Fax +41 (0)31 323 82 27 e-mail: [email protected] Layout Müge Yildirim, ALP Publication frequency Several times yearly at irregular intervals ISSN 1660-7856 (online) 2 Marie-Therese Fröhlich-Wyder and Hans-Peter Bachmann SWISS CHEESE 1 Introduction Swiss-type cheeses were originally manufactured in the Em- Today, Emmental-type cheese (Fig. -

A Guide to Kowalski's Specialty Cheese Read
Compliments of Kowalski’s WWW.KOWALSKIS.COM A GUIDE TO ’ LOCALOUR FAVORITE CHEESES UNDERSTANDING CHEESE TYPES ENTERTAINING WITH CHEESE CHEESE CULTURES OF THE WORLD A PUBLICATION WRITTEN AND PRODUCED BY KOWALSKI’S MARKETS Printed November 2015 SPECIALTY CHEESE EXPERIENCE or many people, Kowalski’s Specialty Cheese Department Sadly, this guide could never be an all-inclusive reference. is their entrée into the world of both cheese and Kowalski’s Clearly there are cheese types and cheesemakers we haven’t Fitself. Many a regular shopper began by exclusively shopping mentioned. Without a doubt, as soon as this guide goes to this department. It’s a tiny little microcosm of the full print, our cheese selection will have changed. We’re certainly Kowalski’s experience, illustrating oh so well our company’s playing favorites. This is because our cheese departments are passion for foods of exceptional character and class. personal – there is an actual person in charge of them, one Cheese Specialist for each and every one of our 10 markets. When it comes to cheese, we pay particular attention Not only do these specialists have their own faves, but so do to cheeses of unique personality and incredible quality, their customers, which is why no two cheese sections look cheeses that are perhaps more rare or have uncommon exactly the same. But though this special publication isn’t features and special tastes. We love cheese, especially local all-encompassing, it should serve as an excellent tool for cheeses, artisanal cheeses and limited-availability treasures. helping you explore the world of cheese, increasing your appreciation and enjoyment of specialty cheese and of that Kowalski’s experience, too. -

Split Defect and Secondary Fermentation in Swiss-Type Cheeses - a Review David F.M
Split defect and secondary fermentation in Swiss-type cheeses - A review David F.M. Daly, Paul L.H. Mcsweeney, Jeremiah J. Sheehan To cite this version: David F.M. Daly, Paul L.H. Mcsweeney, Jeremiah J. Sheehan. Split defect and secondary fermentation in Swiss-type cheeses - A review. Dairy Science & Technology, EDP sciences/Springer, 2010, 90 (1), 10.1051/dst/2009036. hal-00895710 HAL Id: hal-00895710 https://hal.archives-ouvertes.fr/hal-00895710 Submitted on 1 Jan 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Dairy Sci. Technol. 90 (2010) 3–26 Available online at: © INRA, EDP Sciences, 2009 www.dairy-journal.org DOI: 10.1051/dst/2009036 Review Split defect and secondary fermentation in Swiss-type cheeses – A review 1,2 2 1 David F.M. DALY , Paul L.H. McSWEENEY , Jeremiah J. SHEEHAN * 1 Moorepark Food Research Centre, Teagasc, Fermoy, Co. Cork, Ireland 2 Department of Food and Nutritional Sciences, University College, Cork, Ireland Received 15 December 2008 – Revised 25 May 2009 – Accepted 4 September 2009 Published online 16 October 2009 Abstract – Split and secondary fermentation defects in Swiss-type cheese varieties are manifested as undesirable slits or cracks that may lead to downgrading of the cheese. -

Microbiological Risk Assessment of Raw Milk Cheese
Microbiological Risk Assessment of Raw Milk Cheese Risk Assessment Microbiology Section December 2009 MICROBIOLOGICAL RISK ASSESSMENT OF RAW MILK CHEESES ii TABLE OF CONTENTS ACKNOWLEDGEMENTS ................................................................................................. VII ABBREVIATIONS ............................................................................................................. VIII 1. EXECUTIVE SUMMARY .................................................................................................. 1 2. BACKGROUND ................................................................................................................... 8 3. PURPOSE AND SCOPE ..................................................................................................... 9 3.1 PURPOSE...................................................................................................................... 9 3.2 SCOPE .......................................................................................................................... 9 3.3 DEFINITION OF RAW MILK CHEESE ............................................................................... 9 3.4 APPROACH ................................................................................................................ 10 3.5 OTHER RAW MILK CHEESE ASSESSMENTS .................................................................. 16 4. INTRODUCTION .............................................................................................................. 18 4.1 CLASSIFICATION -
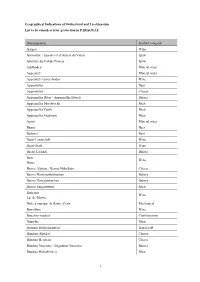
Geographical Indications of Switzerland and Liechtenstein List to Be Considered for Protection in PARAGUAY
Geographical Indications of Switzerland and Liechtenstein List to be considered for protection in PARAGUAY Denomination Product category Aargau Wine Abricotine / Eau-de-vie d’abricot du Valais Spirit Absinthe du Val-de-Travers Spirit Adelboden Mineral water Appenzell Mineral water Appenzell Ausserrhoden Wine Appenzeller Beer Appenzeller Cheese Appenzeller Biber / Appenzeller Biberli Bakery Appenzeller Mostbröckli Meat Appenzeller Pantli Meat Appenzeller Siedwurst Meat Aproz Mineral water Baarer Beer Badener Beer Basel-Landschaft Wine Basel-Stadt Wine Basler Läckerli Bakery Bern Wine Berne Berner Alpkäse / Berner Hobelkäse Cheese Berner Haselnusslebkuchen Bakery Berner Honiglebkuchen Bakery Berner Zungenwurst Meat Bielersee Wine Lac de Bienne Boîte à musique de Sainte-Croix Mechanical Bonvillars Wine Bouchon vaudois Confectionery Boutefas Meat Brienzer Holzschnitzerei Handicraft Bündner Alpkäse Cheese Bündner Bergkäse Cheese Bündner Nusstorte / Engadiner Nusstorte Bakery Bündner Rohschinken Meat 1 Bündner Salsiz / Bündner Doppelsalsiz Meat Bündnerfleisch Meat Calamin Wine Calanda Beer Cardon épineux genevois Vegetable Cervelas Meat Chablais Wine Château de Choully Wine Château de Collex Wine Château du Crest Wine Cheyres Wine Coppa del Ticino Meat Coteau de Bossy Wine Coteau de Bourdigny Wine Coteau de Chevrens Wine Coteau de Choulex Wine Coteau de Choully Wine Coteau de Genthod Wine Coteau de la vigne blanche Wine Coteau de Lully Wine Coteau de Peissy Wine Coteau des Baillets Wine Coteaux de Dardagny Wine Coteaux de Peney Wine Côtes de -

Imported Products and Ingredients
PRODUCT CATALOG • IMPORTED PRODUCTS • INGREDIENTS WORLD-CLASS FOODS DESTINATION IMPORTED BY KCG CORPORATION CHEESE CHEESE ARLA 20000020 20000011 MOZZARELLA PIZZA TOPPING TEX MEX KIDS STICK MOZZARELLA CHEESE CHUNK CHEESE SHREDDED CHEESE SHREDDED CHEESE SHREDDED PACKING : 18 X 6 X 18 G PACKING : 12 X 200 G PACKING : 12 X 175 G PACKING : 12 X 175 G PACKING : 12 X 175 G PAMELLANO 20000045 20000043 20000046 20000044 CHEESE SHREDDED NATURAL HAVARTI NATURAL GOUDA NATURAL EMMENTAL NATURAL MOZZARELLA PACKING : 12 X 100 G CHEESE SLICES CHEESE SLICES CHEESE SLICES CHEESE SLICES PACKING : 14 X 150 G PACKING : 14 X 150 G PACKING : 14 X 150 G PACKING : 14 X 150 G NATURAL CHEDDAR 20000026 FETA 20000032 20000031 CHEESE SLICES APETINA FETA HERBS & SPICES APETINA FETA CHEESE APETINA FETA CHEESE PACKING : 14 X 150 G CHEESE IN BRINE IN JAR IN OLIVE OIL IN HERBS & SPICES PACKING : 6 X 200 G PACKING : 6 X 265 G PACKING : 10 X 100 G PACKING : 10 X 100 G GOUDA HAVARTI 20000030 20000037 20001861 BLOCK CHEESE BLOCK CHEESE CHEESE TRIANGLES CHEESE TRIANGLES VIKING DANISH PACKING : 12 X 200 G PACKING : 12 X 200 G PACKING : 10 X 140 G (ASSORTED FLAVOURS) BLUE CHEESE PACKING : 10 X 140 G PACKING : 8 X 7 X 100 G CHEESE CANTOREL BRIE (APPROX.) PACKING 20000359 : 1 X 3.1 KG 20000360 : 2 X 1.2 KG COOMBE CASTLE 20002025 20002026 20002027 20002028 20002029 COOMBE CASTLE COOMBE CASTLE COOMBE CASTLE COOMBE CASTLE COOMBE CASTLE RED LEICESTER DOUBLE GLOUCESTER LANCASHIRE EXTRA MATURE CHEDDAR DORSET DRUM (DOP) PACKING : 200 G/12 PACKING : 200 G/12 PACKING : 200 G/12 PACKING : -

Characterization of Propionibacteria in Swiss Raw Milk by Biochemical and Molecular-Biological Methods
Research Collection Doctoral Thesis Characterization of propionibacteria in Swiss raw milk by biochemical and molecular-biological methods Author(s): Fessler, Denise Sophie Publication Date: 1997 Permanent Link: https://doi.org/10.3929/ethz-a-001855109 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 12328 Characterisation of propionibacteria in Swiss raw milk by biochemical and molecular-biological methods A thesis submitted to the Swiss Federal Institute of Technology (ETH), Zurich for the degree of Doctor of Technical Sciences presented by DENISE SOPHIE FESSLER Dipl. Lm.-lng. ETH born September 25th, 1969 citizen of Walzenhausen (AR) accepted on the recommendation of Prof. Dr. Z. Puhan, examiner Dr. M. G. Casey, co-examiner Dr. S. Lortal, co-examiner Zurich 1997 To my parents Acknowledgements I would like to express my sincere gratitude to Prof. Dr. Z. Puhan for giving me the chance to carry out this thesis and for his supervision. Very special and warm thanks belong to Dr. M. G. Casey for supporting me. He gave me invaluable suggestions and always had an open ear for my problems. Without his patience and confidence this thesis would not have been possible. I thank Dr. Sylvie Lortal for taking over the external examination of this work. I would like to extend my appreciation to the whole Department of Bio¬ chemistry, FAM. I thank Dr. M. Furst, Dr. J. Jimeno, Dr. A. Baer and Dr. J. Meyer for their backing and Mrs. -

SWISS Cheese Pavilion at Anuga - Media Release (Trend Topics)
SWISS Cheese Pavilion at Anuga - Media Release (Trend Topics) SWISS Cheese Pavilion - Innovation based on tradition At the SWISS Cheese Pavilion (Hall 10.1/ Booth B-021 - D-040), which is organized by Switzerland Cheese Marketing AG in conjunction with Switzerland Global Enterprise, the Swiss dairy industry will be showcasing traditional products of the traditional Swiss export industry over an area of some 900 m2. The high level of Swiss quality awareness and the wealth of practical knowledge as regards processing are equally important for commercial and industrial companies. At the SWISS Cheese Pavilion, three major companies – Emmi Schweiz AG, Mifroma SA and Züger AG – will be showcasing their diverse range of products and services. Eight companies will be presenting their quality product ranges at the joint stand: Alp Senn AG, Bodensee Käse AG, Cremo SA – von Mühlenen, Geska AG, InterCheese AG, Lustenberger & Dürst SA, Margot Fromage SA and the Swiss Association of Soft and Semi-hard Cheese Producers (SGWH). The Swiss cheese classics Appenzeller, Emmental, Gruyère and Tête de Moine will also be represented at the venue and of course offered up for tasting by the Anuga experts. Züger Frischkäse AG - A family business with a global clientèle The family firm Züger Frischkäse AG has been exporting more than 50% of its products to Europe and overseas for many years now. IQF (Individual Quick Frozen) products are primarily offered for system catering outside Europe. “Thanks to their long shelf-life, they can easily be transported by ship, which is cheaper than doing so by plane,” says Christoph Scherrer, sales manager at Züger Frischkäse AG. -

Rapport Annuel 2018 Switzerland Cheese Marketing SA VÖGELI AG Tel.VÖGELI +41 (0)34 AG 409 10 10 Tel
Rapport annuel 2018 Switzerland Cheese Marketing SA VÖGELI AG Tel.VÖGELI +41 (0)34 AG 409 10 10 Tel. +41 (0)34 409 10 10 Marketingproduktion & Druck FaxMarketingproduktion +41 (0)34 409 10 15& Druck Fax +41 (0)34 409 10 15 Sägestrasse 21–23 www.voegeli.chSägestrasse 21–23 www.voegeli.ch CH-3550 Langnau i. E. [email protected] Langnau i. E. [email protected] Éditorial L’exercice sous revue s’est caractérisé par mage Suisse. Car il marie tous les sels de et régional sont reconnus, tout comme sa la prise de décisions importantes pour nos vies.», est axé sur les plus-values du qualité. Ces avantages expliquent pour- l’avenir du fromage suisse. Ainsi, les fu- fromage suisse et il fait le lien avec les quoi, ces derniers temps, nous avons turs rapports de propriété des cultures valeurs personnelles des groupes cibles. observé une intensification des tentatives dites de Liebefeld ont pu être réglés à la Il vise désormais en première ligne des d’exploiter ces valeurs pour promouvoir fin novembre 2018, en présence du consommatrices et des consommateurs des produits de substitution qui ne sont conseiller fédéral Johann Schneider- citadins et jeunes, c’est-à-dire des per- pas du fromage, leur ingrédient principal Ammann. La reproduction des cultures sonnes qui ne savent plus, ou pas en- n’étant pas du lait. Chez SCM, nous sui- fromagères suisses repose désormais core, tout ce qu’il y a de précieux dans un vons de près cette évolution et interve- sur une nouvelle base grâce à la création, morceau de fromage suisse.