1 Are We Ready for Genome Editing in Human Embryos for CLINICAL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Discovery of DNA Structure and Function: Watson and Crick By: Leslie A
01/08/2018 Discovery of DNA Double Helix: Watson and Crick | Learn Science at Scitable NUCLEIC ACID STRUCTURE AND FUNCTION | Lead Editor: Bob Moss Discovery of DNA Structure and Function: Watson and Crick By: Leslie A. Pray, Ph.D. © 2008 Nature Education Citation: Pray, L. (2008) Discovery of DNA structure and function: Watson and Crick. Nature Education 1(1):100 The landmark ideas of Watson and Crick relied heavily on the work of other scientists. What did the duo actually discover? Aa Aa Aa Many people believe that American biologist James Watson and English physicist Francis Crick discovered DNA in the 1950s. In reality, this is not the case. Rather, DNA was first identified in the late 1860s by Swiss chemist Friedrich Miescher. Then, in the decades following Miescher's discovery, other scientists--notably, Phoebus Levene and Erwin Chargaff--carried out a series of research efforts that revealed additional details about the DNA molecule, including its primary chemical components and the ways in which they joined with one another. Without the scientific foundation provided by these pioneers, Watson and Crick may never have reached their groundbreaking conclusion of 1953: that the DNA molecule exists in the form of a three-dimensional double helix. The First Piece of the Puzzle: Miescher Discovers DNA Although few people realize it, 1869 was a landmark year in genetic research, because it was the year in which Swiss physiological chemist Friedrich Miescher first identified what he called "nuclein" inside the nuclei of human white blood cells. (The term "nuclein" was later changed to "nucleic acid" and eventually to "deoxyribonucleic acid," or "DNA.") Miescher's plan was to isolate and characterize not the nuclein (which nobody at that time realized existed) but instead the protein components of leukocytes (white blood cells). -

Jewels in the Crown
Jewels in the crown CSHL’s 8 Nobel laureates Eight scientists who have worked at Cold Max Delbrück and Salvador Luria Spring Harbor Laboratory over its first 125 years have earned the ultimate Beginning in 1941, two scientists, both refugees of European honor, the Nobel Prize for Physiology fascism, began spending their summers doing research at Cold or Medicine. Some have been full- Spring Harbor. In this idyllic setting, the pair—who had full-time time faculty members; others came appointments elsewhere—explored the deep mystery of genetics to the Lab to do summer research by exploiting the simplicity of tiny viruses called bacteriophages, or a postdoctoral fellowship. Two, or phages, which infect bacteria. Max Delbrück and Salvador who performed experiments at Luria, original protagonists in what came to be called the Phage the Lab as part of the historic Group, were at the center of a movement whose members made Phage Group, later served as seminal discoveries that launched the revolutionary field of mo- Directors. lecular genetics. Their distinctive math- and physics-oriented ap- Peter Tarr proach to biology, partly a reflection of Delbrück’s physics train- ing, was propagated far and wide via the famous Phage Course that Delbrück first taught in 1945. The famous Luria-Delbrück experiment of 1943 showed that genetic mutations occur ran- domly in bacteria, not necessarily in response to selection. The pair also showed that resistance was a heritable trait in the tiny organisms. Delbrück and Luria, along with Alfred Hershey, were awarded a Nobel Prize in 1969 “for their discoveries concerning the replication mechanism and the genetic structure of viruses.” Barbara McClintock Alfred Hershey Today we know that “jumping genes”—transposable elements (TEs)—are littered everywhere, like so much Alfred Hershey first came to Cold Spring Harbor to participate in Phage Group wreckage, in the chromosomes of every organism. -

Centrosome Detection in Sea Urchin Eggs with a Monoclonal
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 8488-8492, December 1987 Cell Biology Centrosome detection in sea urchin eggs with a monoclonal antibody against Drosophila intermediate filament proteins: Characterization of stages of the division cycle of centrosomes (cytoskeleton/fertilization/microtubules/mitosis) HEIDE SCHATTEN*, MARIKA WALTERt, DANIEL MAZIAt, HARALD BIESSMANNt, NEIDHARD PAWELETZ§, GE2RARD COFFE*, AND GERALD SCHATTEN* *Integrated Microscopy Resource for Biomedical Research, University of Wisconsin, 1117 West Johnson Street, Madison, WI 53706; tCenter for Developmental Biology, University of California, Irvine, CA 92717; tHopkins Marine Station, Department of Biological Sciences, Stanford University, Pacific Grove, CA 93950; and 1lnstitute for Cell and Tumor Biology, German Cancer Research Center, D-6900 Heidelberg, Federal Republic of Germany Contributed by Daniel Mazia, August 27, 1987 ABSTRACT A mouse monoclonal antibody generated Most all of the previous immunocytochemical work on against DrosophUa intermediate filament proteins (designated centrosomes has used an autoimmune serum from a patient Ah6/5/9 and referred to herein as Ah6) is found to cross-react suffering from CREST (calcinosis, Raynaud phenomenon, specifically with centrosomes in sea urchin eggs and with a esophageal dysmotility, sclerodactyly, telangiectasia) scle- 68-kDa antigen in eggs and isolated mitotic apparatus. When roderma (6-10). However, the inability to use this serum for preparations stained with Ah6 are counterstained with a immunoblotting precluded -
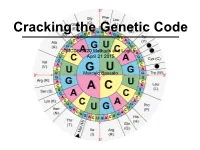
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J. -

Bioelectric Responses at Fertilization: Separation of the Events Associated
Gamete Research 5:363-377 (1982) Bioelectric Responses at Fertilization: Separation of the Events Associated With Insemination From Those Due to the Cortical Reaction in Sea Urchin, Lytechinus variegatus Dieter Hulser and Gerald Schatten Department of Biological Science, The Florida State University, Tallahassee The bioelectric responses at fertilization of the sea urchin Lytechinus variegatus are a com- plex series of membrane potential and resistance changes that occur concomitant with ga- mete fusion, ionic fluxes, and the cortical granule discharge. This work attempts to separate the electrical effects of sperm-egg interactions from those of the cortical reactions. Two ap- proaches were taken to discern the electrical events associated with insemination, distinct from cortical granule discharge: 1) fertilization of eggs treated with 3% urethane, 10 mM procaine, or 10 mM nicotine, to prevent the cortical reaction and 2) refertilization of fertil- ized eggs (denuded with 1 mM aminotriazole containing 1 mg/ml soybean trypsin inhibitor). Cortical granule discharge in the absence of sperm incorporation was investigated by artifi- cial activation with 5 pM A23187 or by fertilization in the presence of 10 pM cytochalasin D, which prevents incorporation. These results are consistent with a model in which the sperm-egg interaction triggers both a rapid (50-400 msec), but minor (= 10 mV), electrical transient that leads to an action potential and then both the Na+-dependent fast block to polyspermy and the late block re- sulting from the secretion of the cortical granules. Key words: fertilization, sea urchin, bioelectric response, secretion, motility INTRODUCTION At fertilization the sea urchin egg undergoes a complex series of electrical changes in membrane potential and resistance. -

Fraudulent Human Embryonic Stem Cell Research in South Korea: Lessons Learned
Accountability in Research, 13:101–109, 2006 Copyright © Taylor & Francis Group, LLC ISSN: 0898-9621 print DOI: 10.1080/08989620600634193 GACR0898-96211545-5815Accountability in Research:Research Policies and Quality Assurance, Vol. 13, No. 01, February 2006: pp. 0–0 Commentary FRAUDULENT HUMAN EMBRYONIC STEM CELL RESEARCH IN SOUTH KOREA: LESSONS LEARNED Commentary:D. B. Resnik et Korean al. Stem Cell Fraud DAVID B. RESNIK , JD, PHD National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, North Carolina, USA ADIL E. SHAMOO, PHD Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine, Baltimore, Maryland, USA SHELDON KRIMSKY, PHD Department of Urban & Environmental Policy & Planning, Tufts University, Boston, Massachusetts, USA Now that most of the smoke has cleared from the South Korean human embryonic stem cell fraud, it is time to reflect on some lessons that one can learn from this scandal. First, a brief review of events will help to set the stage. In June 2005, Seoul University investigator Woo Suk Hwang and 24 co-authors published what appeared to be a ground- breaking paper in Science in which they claimed to have estab- lished eleven embryonic stem cell lines containing nuclear DNA from somatic cells of research subjects (Hwang et al., 2005). In March 2004, Hwang’s research team had published another apparently important paper in which they claimed to have estab- lished one cell line with the nuclear DNA from a research subject (Hwang et al 2004). If these two papers had been valid, they would have represented a significant step forward in human embryonic stem cell research, since they would have demon- strated the feasibility of a technique known as therapeutic Editor’s note: Although this piece is not related to the topic of this issue, we felt it was important to comment on the recent events in South Korea. -

In 1953 in England James Watson and Francis Crick Discovered the Structure of DNA in the Now-Famous Scientific Narrative Known As the “Race Towards the Double Helix”
THE NARRATIVES OF SCIENCE: LITERARY THEORY AND DISCOVERY IN MOLECULAR BIOLOGY PRIYA VENKATESAN In 1953 in England James Watson and Francis Crick discovered the structure of DNA in the now-famous scientific narrative known as the “race towards the double helix”. Meanwhile in France, Roland Barthes published his first book, Writing Degree Zero, on literary theory, which became the intellectual precursor for the new human sciences that were developing based on Saussurean linguistics. The discovery by Watson and Crick of the double helix marked a definitive turning point in the development of the life sciences, paving the way for the articulation of the genetic code and the emergence of molecular biology. The publication by Barthes was no less significant, since it served as an exemplar for elucidating how literary narratives are structured and for formulating how textual material is constructed. As Françoise Dosse notes, Writing Degree Zero “received unanimous acclaim and quickly became a symptom of new literary demands, a break with tradition”.1 Both the work of Roland Barthes and Watson and Crick served as paradigms in their respective fields. Semiotics, the field of textual analysis as developed by Barthes in Writing Degree Zero, offered a new direction in the structuring of narrative whereby each distinct unit in a story formed a “code” or “isotopy” that categorizes the formal elements of the story. The historical concurrence of the discovery of the double helix and the publication of Writing Degree Zero may be mere coincidence, but this essay is an exploration of the intellectual influence that both events may have had on each other, since both the discovery of the double helix and Barthes’ publication gave expression to the new forms of knowledge 1 Françoise Dosse, History of Structuralism: The Rising Sign, 1945-1966, trans. -

Strategies for Improving Animal Models for Regenerative Medicine
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Cell Stem Cell Forum Strategies for Improving Animal Models for Regenerative Medicine Jose Cibelli,1 Marina E. Emborg,2 Darwin J. Prockop,3 Michael Roberts,4 Gerald Schatten,5 Mahendra Rao,6 John Harding,7 and Oleg Mirochnitchenko7,* 1Michigan State University, Cellular Reprogramming Laboratory, Department of Animal Science, B270 Anthony Hall, East Lansing, MI 48824, USA 2University of Wisconsin-Madison, Department of Medical Physics and Wisconsin National Primate Research Center, 1223 Capitol Court, Madison, WI 53715, USA 3Texas A&M Health Science Center, College of Medicine Institute for Regenerative Medicine at Scott and White, Department of Medicine, 5701 Airport Road, Module C, Temple, TX 76502, USA 4University of Missouri, 240b C.S. Bond Life Sciences Center, 1201 East Rollins Street, Columbia, MO 65211-7310, USA 5University of Pittsburgh, Department of Cell Biology and Physiology, S362 Biomedical Science Towers, 3500 Terrace Street, Pittsburgh, PA 15261, USA 6Center for Regenerative Medicine, National Institutes of Health, 50 South Drive, Suite 1140, Bethesda, MD 20892, USA 7Division of Comparative Medicine/ORIP/DPCPSI/OD, National Institutes of Health, 6701 Democracy Boulevard, Suite 943/950, Bethesda, MD 20892, USA *Correspondence: [email protected] http://dx.doi.org/10.1016/j.stem.2013.01.004 The field of regenerative medicine is moving toward translation to clinical practice. However, there are still knowledge gaps and safety concerns regarding stem cell-based therapies. Improving large animal models and methods for transplantation, engraftment, and imaging should help address these issues, facilitating eventual use of stem cells in the clinic. -

James Watson and Francis Crick
James Watson and Francis Crick https://www.ducksters.com/biography/scientists/watson_and_crick.php biographyjameswatsonandfranciscrick.mp3 Occupation: Molecular biologists Born: Crick: June 8, 1916 Watson: April 6, 1928 Died: Crick: July 28, 2004 Watson: Still alive Best known for: Discovering the structure of DNA Biography: James Watson James Watson was born on April 6, 1928 in Chicago, Illinois. He was a very intelligent child. He graduated high school early and attended the University of Chicago at the age of fifteen. James loved birds and initially studied ornithology (the study of birds) at college. He later changed his specialty to genetics. In 1950, at the age of 22, Watson received his PhD in zoology from the University of Indiana. James Watson and Francis Crick https://www.ducksters.com/biography/scientists/watson_and_crick.php James D. Watson. Source: National Institutes of Health In 1951, Watson went to Cambridge, England to work in the Cavendish Laboratory in order to study the structure of DNA. There he met another scientist named Francis Crick. Watson and Crick found they had the same interests. They began working together. In 1953 they published the structure of the DNA molecule. This discovery became one of the most important scientific discoveries of the 20th century. Watson (along with Francis Crick, Rosalind Franklin, and Maurice Wilkins) was awarded the Nobel Prize in Physiology or Medicine in 1962 for the discovery of the DNA structure. He continued his research into genetics writing several textbooks as well as the bestselling book The Double Helix which chronicled the famous discovery. Watson later served as director of the Cold Spring Harbor Lab in New York where he led groundbreaking research into cancer. -

From Controlling Elements to Transposons: Barbara Mcclintock and the Nobel Prize Nathaniel C
454 Forum TRENDS in Biochemical Sciences Vol.26 No.7 July 2001 Historical Perspective From controlling elements to transposons: Barbara McClintock and the Nobel Prize Nathaniel C. Comfort Why did it take so long for Barbara correspondence. From these and other to prevent her controlling elements from McClintock (Fig. 1) to win the Nobel Prize? materials, we can reconstruct the events moving because their effects were difficult In the mid-1940s, McClintock discovered leading up to the 1983 prize*. to study when they jumped around. She genetic transposition in maize. She What today are known as transposable never had any inclination to pursue the published her results over several years elements, McClintock called ‘controlling biochemistry of transposition. and, in 1951, gave a famous presentation elements’. During the years 1945–1946, at Current understanding of how gene at the Cold Spring Harbor Symposium, the Carnegie Dept of Genetics, Cold activity is regulated, of course, springs yet it took until 1983 for her to win a Nobel Spring Harbor, McClintock discovered a from the operon, François Jacob and Prize. The delay is widely attributed to a pair of genetic loci in maize that seemed to Jacques Monod’s 1960 model of a block of combination of gender bias and gendered trigger spontaneous and reversible structural genes under the control of an science. McClintock’s results were not mutations in what had been ordinary, adjacent set of regulatory genes (Fig. 2). accepted, the story goes, because women stable alleles. In the term of the day, they Though subsequent studies revealed in science are marginalized, because the made stable alleles into ‘mutable’ ones. -

Cover June 2011
z NOBEL LAUREATES IN Qui DNA RESEARCH n u SANGRAM KESHARI LENKA & CHINMOYEE MAHARANA F 1. Who got the Nobel Prize in Physiology or Medicine 1933) for discovering the famous concept that says chromosomes carry genes? a. Gregor Johann Mendel b. Thomas Hunt Morgan c. Aristotle d. Charles Darwin 5. Name the Nobel laureate (1959) for his discovery of the mechanisms in the biological 2. The concept of Mutations synthesis of ribonucleic acid and are changes in genetic deoxyribonucleic acid? information” awarded him a. Arthur Kornberg b. Har Gobind Khorana the Nobel Prize in 1946: c. Roger D. Kornberg d. James D. Watson a. Hermann Muller b. M.F. Perutz c. James D. Watson 6. Discovery of the DNA double helix fetched them d. Har Gobind Khorana the Nobel Prize in Physiology or Medicine (1962). a. Francis Crick, James Watson, Rosalind Elsie Franklin b. Francis Crick, James Watson and Maurice Willkins c. James Watson, Maurice Willkins, Rosalind Elsie Franklin 3. Identify the discoverer and d. Maurice Willkins, Rosalind Elsie Franklin and Francis Crick Nobel laureate of 1958 who found DNA in bacteria and viruses. a. Louis Pasteur b. Alexander Fleming c. Joshua Lederberg d. Roger D. Kornberg 4. A direct link between genes and enzymatic reactions, known as the famous “one gene, one enzyme” hypothesis, was put forth by these 7. They developed the theory of genetic regulatory scientists who shared the Nobel Prize in mechanisms, showing how, on a molecular level, Physiology or Medicine, 1958. certain genes are activated and suppressed. Name a. George Wells Beadle and Edward Lawrie Tatum these famous Nobel laureates of 1965. -

Biographical Notes on Scientists Involved in the Asilomar Process M.J
International Dimensions of Ethics Education in Science and Engineering Case Study Series: Asilomar Conference on Laboratory Precautions Appendix D: Biographical Notes on Scientists involved in the Asilomar Process M.J. Peterson Version 1, June 2010 Edward A. Adelberg (1920-2009). PhD Yale 1949. Chair of Yale Department of Microbiology 1961-64 and 1970-72; a founding member of Yale Department of Genetics. Deputy Provost for the Biomedical Sciences, 1983-91. Specialist in plasmid biochemistry of E. coli. Ephraim Anderson (1911-2006). M.D., Durham University. Served in the Royal Army Medical Corps during World War II where he developed interests in Epidemiology. Researcher in Enteric Laboratory of the British Public Health Laboratory Service 1947, Deputy Director 1952, Director 1954-1978. Came to public notice for tracing sources of typhoid outbreaks in Zermatt (1963) and Aberdeen (1964). Built on earlier work by Japanese researchers to demonstrate the plasmid-based pathways by which bacteria could spread antibiotic resistance to others and became the world’s leading expert on antibiotic resistance. Also prominent in efforts to limit use of antibiotics in raising animals. Fellow of the Royal Society 1968; Companion of the Order of the British Empire 1976. Eric Ashby, Baron Ashby (1904-1992). Lecturer in Botany, Imperial College London 1931-35; Reader in Botany Bristol University 1935-37; Professor of Botany University of Sydney 1938-1946; Chair of Botany, University of Manchester 1947-50. Turned to administration as President and Vice-Chancellor of Queen's University, Belfast 1950-59; Master of Clare College in Cambridge University 1959-67 and Vice-Chancellor of Cambridge University 1967-1969.